PMS synthesis method
A synthesis method and technology of mixed solvents are applied in the field of PMS synthesis to achieve the effects of simple process, avoiding side reactions and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
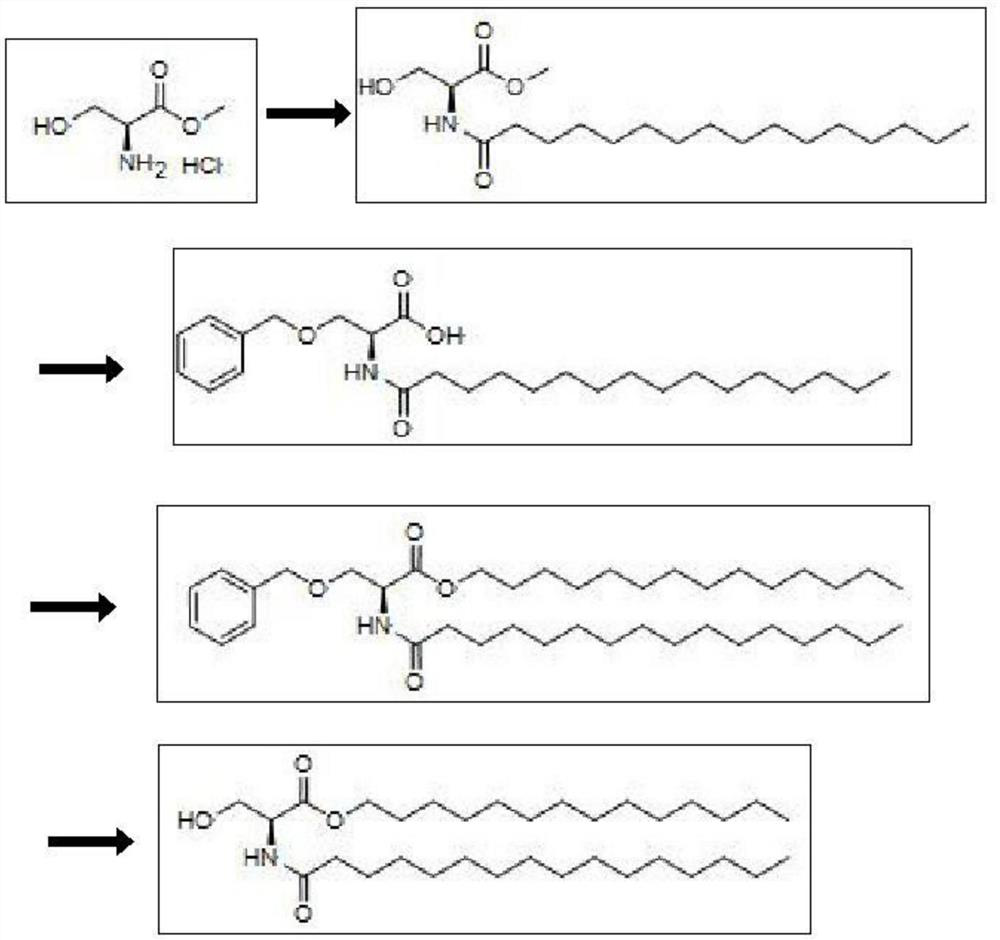
[0047] Example 1: Synthesis of N-palmitoyl-L-serine methyl ester
[0048] Add 100.0g L-serine methyl ester hydrochloride, 700.0g tetrahydrofuran, and 126.6g triethylamine to the four-necked flask successively, and stir at room temperature; then dissolve 176.6g palmitoyl chloride in 300g tetrahydrofuran, and slowly dropwise add A solution of the palmitoyl chloride in tetrahydrofuran. After dropping, stir the reaction at room temperature for 1-2 hours. After the reaction was completed, 2000 g of water was slowly added dropwise to the system to precipitate a large amount of white solid, which was collected by filtration to obtain 218.3 g of the product N-palmitoyl-L-serine methyl ester, with a yield of 95.5%.
Embodiment 2
[0049] Example 2: Synthesis of N-palmitoyl-O-benzyl-L-serine
[0050] Under continuous nitrogen purging, add 36.63g of sodium hydrogen (60% content) to the four-neck flask successively, add dropwise 655.0g of tetrahydrofuran, stir at room temperature, and slowly add 218.3g of N-palmitoyl-L-serine in batches at this temperature Methyl ester, stirred at room temperature for 0.5h to drive off the generated hydrogen. Then, under the protection of nitrogen, 152.3 g of benzyl bromide was added dropwise, and after the drop was completed, the reaction was stirred at room temperature for 1-2 hours. After the reaction is completed, add 488.4g of 10wt% aqueous sodium hydroxide solution dropwise to the system, and stir at room temperature for 3-5 hours. After the reaction is completed, adjust the pH to 3-4 with hydrochloric acid, and extract 3 times with ethyl acetate (100g*3). The organic layers were combined, concentrated to dryness under reduced pressure, and recrystallized from metha...
Embodiment 3
[0051] Example 3: Synthesis of N-palmitoyl-O-benzyl-L-serine tetradecyl ester
[0052] Add 243.6g N-palmitoyl-O-benzyl-L-serine, 600g dichloromethane, 2.05gDMF successively in the four-necked flask, stir at room temperature, drop 85.6g oxalyl chloride at this temperature, stir at room temperature and react 2- 3 hours. After the reaction was completed, the obtained acid chloride was set aside for use, and then 132.5 g of tetradecyl alcohol, 600.0 g of dichloromethane, and 85.3 g of triethylamine were added to another four-necked flask. Under nitrogen protection, the aforementioned acid chloride was added dropwise at room temperature, 1-2 After hours of dripping, continue to react for 2-3 hours. After the reaction was completed, it was washed with water (100g*3) for 3 times to obtain an organic layer, which was concentrated to dryness under reduced pressure to obtain the crude N-palmitoyl-O-benzyl-L-serine tetradecyl ester, which was directly For the next step, the yield is 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com