Preparation method of chiral 1-tert-butyl-3-methyl-6-methylpiperazine-1, 3-diformate
A technology of methylpiperazine and diformate, applied in the field of organic compound synthesis, can solve the problems of low yield, high price of palladium carbon, unfavorable industrial production, etc., and achieves reduction of production cost, easy availability of raw materials, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
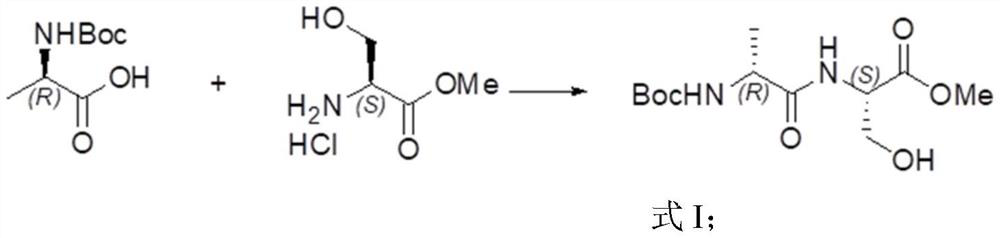
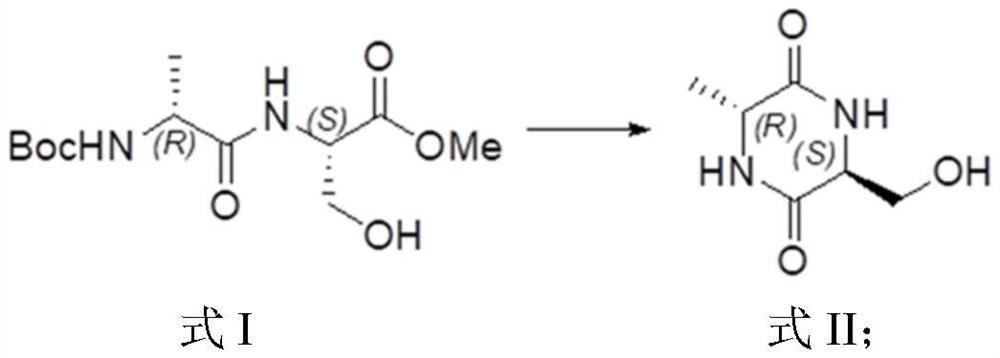
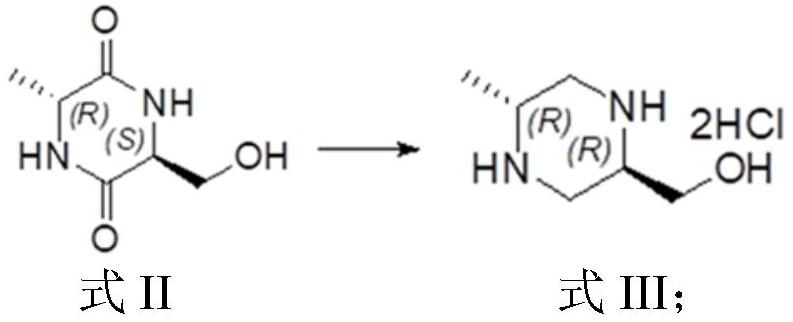
[0136] This example provides a method for preparing (3R,6R)-1-tert-butyl-3-methyl-6-methylpiperazine-1,3-dicarboxylate, which specifically includes the following steps:
[0137] (1)
[0138]Boc-D-alanine (183.5g, 0.97mol) was added to 2.5L of dichloromethane, and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide was added dropwise under ice-cooling ( 205g, 1.07mol), stirred for 10min, then added dropwise L-serine methyl ester hydrochloride (158.7g, 1.02mol) and triethylamine (161mL, 1.16mol), naturally warmed to room temperature, and reacted for 10h; Add 3L of pure water, let it stand, and separate the liquids to obtain an organic phase and an aqueous phase. The aqueous phase is extracted once with 1L of dichloromethane, and the organic phases are combined, and successively passed through hydrochloric acid with a gram equivalent concentration of 1N, saturated sodium bicarbonate, pure Wash with water and saturated brine, dry over anhydrous sodium sulfate, and concentrate to obta...
Embodiment 2
[0158] This example provides a method for preparing (3R,6R)-1-tert-butyl-3-methyl-6-methylpiperazine-1,3-dicarboxylate, which specifically includes the following steps:
[0159] (1)
[0160] Add Boc-D-alanine (189g, 1mol) to 2.5L dichloroethane, add dicyclohexylcarbodiimide (206g, 1mol) dropwise under ice-cooling, stir for 5min, then add L-serine dropwise Methyl ester hydrochloride (176.8g, 1.14mol) and N-methylmorpholine (148mL, 1.7mol) were naturally warmed to room temperature and reacted for 9.5h; 3L of pure water was added to the reaction system, allowed to stand and separated, The organic phase and the aqueous phase were obtained, the aqueous phase was extracted once with 1L dichloroethane, the organic phases were combined and washed successively with 1N hydrochloric acid, saturated sodium bicarbonate, pure water, saturated brine, anhydrous sodium sulfate Dried and concentrated to give 258 g of product , the yield was 89%.
[0161] (2)
[0162] The product (255 g...
Embodiment 3
[0174] This example provides a method for preparing (3R,6R)-1-tert-butyl-3-methyl-6-methylpiperazine-1,3-dicarboxylate, which specifically includes the following steps:
[0175] (1)
[0176] Boc-D-alanine (200g, 1.06mol) was added to 2.5L chloroform, and 2-(7-azabenzotriazole)-N,N,N',N was added dropwise under ice-cooling '-Tetramethylurea hexafluorophosphate (334.6g, 0.88mol), stirred for 15min, then added dropwise L-serine methyl ester hydrochloride (206g, 1.33mol) and diisopropylethylamine (162.4mL, 1.17 mol), naturally warmed to room temperature, and reacted for 10.5h; 3L of pure water was added to the reaction system, left to stand and separated to obtain an organic phase and an aqueous phase, and the aqueous phase was extracted once with 1L of chloroform, and the organic phase was combined and sequentially Washed with 1N hydrochloric acid, saturated sodium bicarbonate, pure water, and saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 280...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com