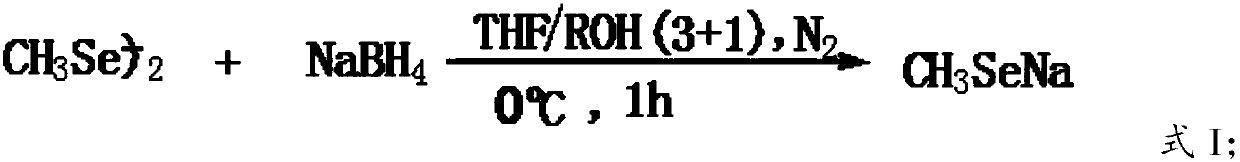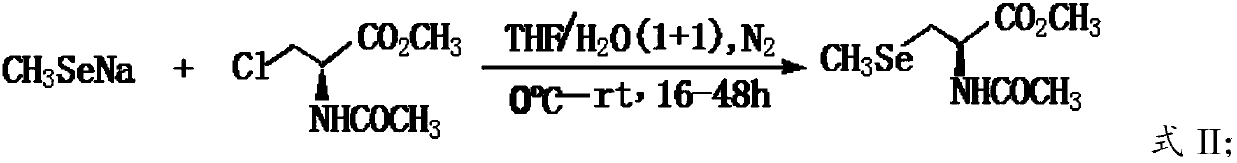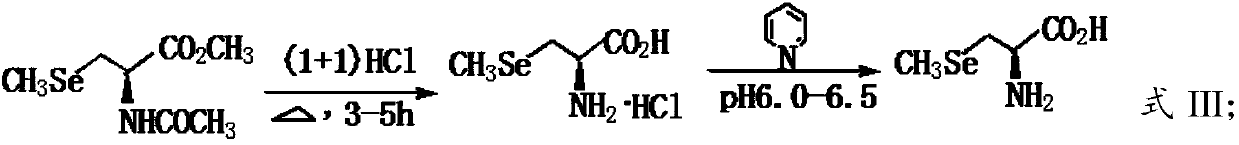Preparation method of L-Se-methylselenocysteine
A technology of selenocysteine and cysteine methyl ester, applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of difficult source of cysteine raw materials, complex synthesis process, serious pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a preparation method of L-selenomethylselenocysteine, which comprises the following steps:
[0034] A) Perform a nucleophilic substitution reaction between sodium methylselenoate and N-acetyl-3-chloro-L-serine methyl ester to obtain N-acetyl-3-selenomethyl-L-selenocysteine methyl ester;
[0035] B) Hydrolyzing the N-acetyl-3-selenomethyl-L-selenocysteine methyl ester obtained in step A) in a hydrochloric acid solution to obtain L-selenomethylselenocysteine.
[0036] In the present invention, preferably under the conditions of ice bath and nitrogen protection, dimethyl diselenide, sodium borohydride and activator are mixed in a solvent and reacted to obtain sodium methyl selenoxide; more preferably, dimethyl diselenide The ether and sodium borohydride are dissolved in the solvent, and then under the conditions of ice bath and nitrogen protection, the activator is added dropwise to obtain sodium methylselenoate.
[0037] The reaction equation of this st...
Embodiment 1
[0058] Use N-acetyl-3-chloro-L-serine methyl ester to prepare L-selenomethylselenocysteine:
[0059] Dissolve 60 grams of dimethyl diselenide and 25 grams of sodium borohydride in 600 mL of THF, cool in an ice bath, and 2 Under protection, start stirring, add 120mL of anhydrous methanol dropwise, after about 0.5h, the dripping process, the reaction is violent, a colorless solution is obtained, after continuing to stir for 0.5h, add 600mL of water, after stirring evenly, slowly add 70.0 G N-acetyl-3-chloro-L-serine methyl ester, after the addition, warm to room temperature naturally, and stir for 12h until the reaction of N-acetyl-3-chloro-L-serine methyl ester is complete (TLC detection), use 6.0MHCl Adjust system pH <5.0, extract with ethyl acetate, combine the extracts, wash with water, dry with anhydrous sodium sulfate, filter, and remove ethyl acetate under reduced pressure to obtain a brown-yellow oily liquid, which is N-acetyl-3-selenomethyl-L-selenium The crude product of ...
Embodiment 2
[0069] Use N-acetyl-3-chloro-L-serine methyl ester to prepare L-selenomethylselenocysteine:
[0070] Dissolve 60 grams of dimethyl diselenide and 25 grams of sodium borohydride in 600 mL of THF, cool in an ice bath, and 2 Under protection, start stirring, add 120mL of anhydrous methanol dropwise, after about 0.5h, the dripping process, the reaction is violent, a colorless solution is obtained, after continuing to stir for 0.5h, add 600mL of water, after stirring evenly, slowly add 80.0 Grams of N-acetyl-2-chloro-L-serine methyl ester, after the addition, warm to room temperature naturally, and stir for 24h until the reaction of N-acetyl-3-chloro-L-serine methyl ester is complete (TLC detection), use 6.0MHCl Adjust system pH <5.0, extract with ethyl acetate, combine the extracts, wash with water, dry with anhydrous sodium sulfate, filter, and remove ethyl acetate under reduced pressure to obtain a brown-yellow oily liquid, which is N-acetyl-3-selenomethyl-L-selenium The crude prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com