Synthesis method of methyl 2-acetylamino-3-chloropropionate
A technology of chloroalanine methyl ester and alanine methyl ester, which is applied in the field of preparation of ramipril intermediates, can solve the problems of low yield and purity, and achieves high reaction yield, few by-products, and high-quality products. excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
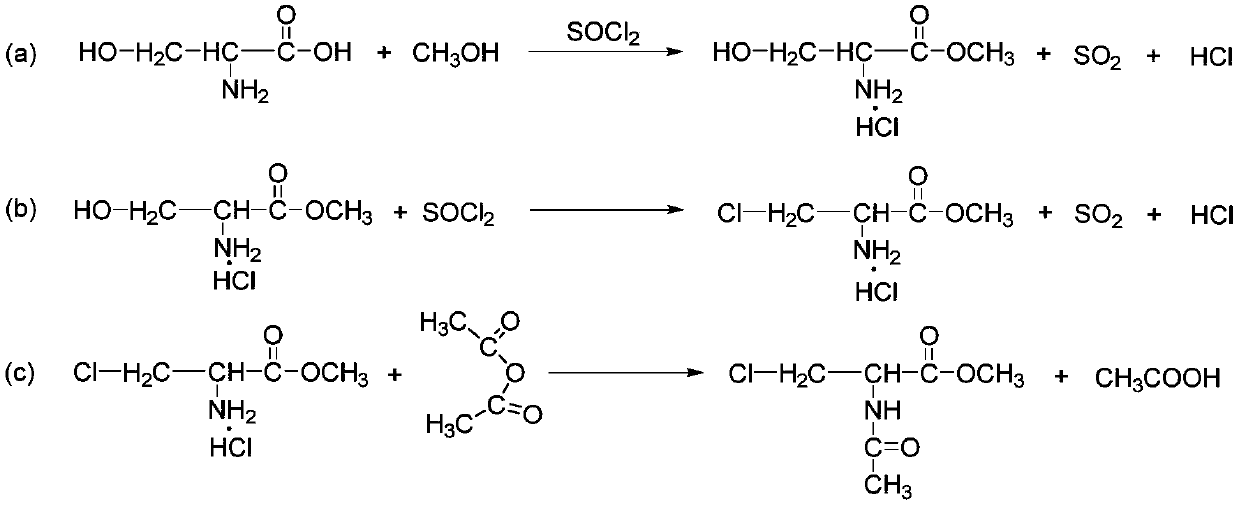
[0015] This embodiment provides a kind of synthetic method of acetylamino-3-chloroalanine methyl ester, comprising the following steps:
[0016] (1) Add L-serine methyl ester hydrochloride into dichloromethane, then dropwise add thionyl chloride A, carry out segmental temperature control reaction at 30-60°C, cool down to 20°C after the reaction, add water Layering, obtaining 3-chloro-L-alanine methyl ester hydrochloride aqueous solution after activated carbon removal of impurities;
[0017] (2) Control the temperature of the above-mentioned 3-chloro-L-alanine methyl ester hydrochloride solution to 15-25°C; add aqueous sodium bicarbonate solution and acetic anhydride dropwise at the same time, and keep warm for 1-4 hours after the addition; Acetylamino-3-chloroalanine methyl ester was obtained after extraction, concentration, recrystallization, centrifugation and drying.
[0018] In the method, the staged temperature control reaction can control the reaction rate of the reacti...
Embodiment 1
[0043] Embodiment 1 of the present invention provides a kind of synthetic method of acetylamino-3-chloroalanine methyl ester, comprising the following steps:
[0044](1) Add 200kg L-serine and 700L methanol into the reaction kettle, cool down to 10°C, and slowly add 330kg of thionyl chloride dropwise; wherein, the temperature of the dropwise addition process is controlled at 12°C; after 10 hours of dropping, the temperature is raised to 38°C, reacted for 48 hours, finally crystallized by cooling, centrifuged and dried to obtain L-serine methyl ester hydrochloride. Wherein, the solvent removed in the centrifugation process is used as the next batch of masterbatch for this reaction.
[0045] (2) Add 90kg of L-serine methyl ester hydrochloride into 700L of dichloromethane, and then dropwise add 100kg of thionyl chloride; after 2 hours of dropping, control the temperature of the reaction system to 30°C, and react for 2 hours. Then raise the temperature to 40°C, react for 3h, then...
Embodiment 2
[0050] Embodiment 2 of the present invention provides a kind of synthetic method of acetylamino-3-chloroalanine methyl ester, comprising the following steps:
[0051] (1) Add 200kg of L-serine, 300L of methanol and 450L of recovered methanol into the reaction kettle, cool down to 10°C, and slowly add 270kg of thionyl chloride dropwise; wherein, the temperature of the dropping process is controlled at 14°C; After completion, the temperature was raised to 38°C, reacted for 48 hours, and finally crystallized by cooling, centrifuged and dried to obtain L-serine methyl ester hydrochloride. Wherein, the solvent removed in the centrifugation process is used as the next batch of masterbatch for this reaction.
[0052] (2) Add 90kg of L-serine methyl ester hydrochloride into 700L of dichloromethane, and then dropwise add 100kg of thionyl chloride; after 2 hours of dropping, control the temperature of the reaction system to 30°C, and react for 2 hours. Then raise the temperature to 40°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com