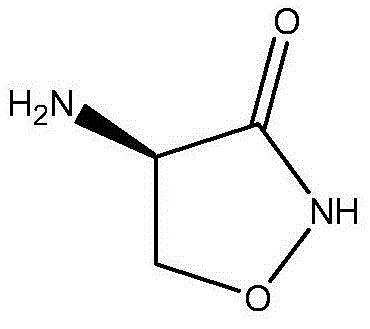
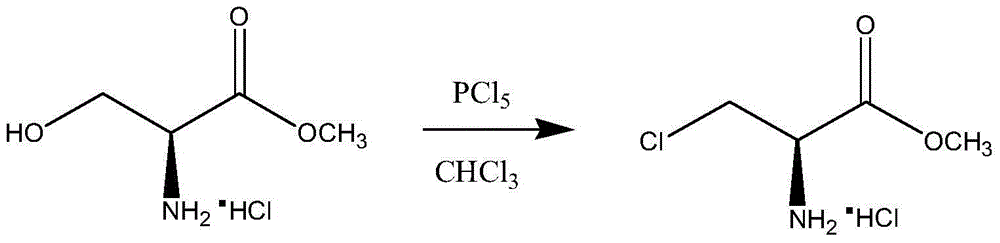
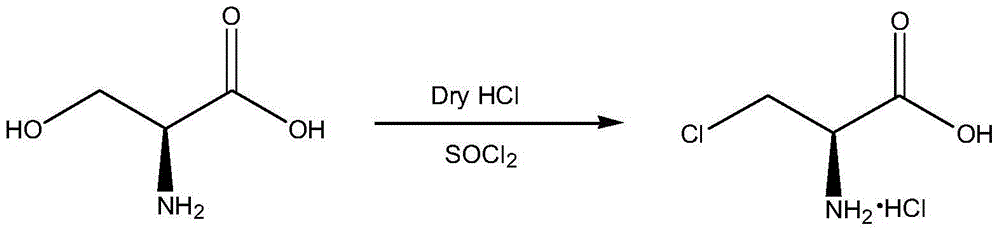
Synthetic method of D-cycloserine intermediate
A technology of serine methyl ester and alanine methyl ester, which is applied in the field of medicine, can solve the problems of high price of thionyl bromide, hidden safety hazards, and low atom economy, so as to improve operational safety, facilitate industrial scale-up, and collect The effect of high rate and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 1L four-neck flask, add 150ml of acetonitrile and 450ml of dichloromethane, weigh 60g of D-serine methyl ester hydrochloride (0.386mol) into the mixed solvent, stir evenly, cool to 0℃, and then slowly add SOCl 2 34.4ml (0.463mol), keep the temperature at 0℃, add SOCl 2 Then the temperature was raised to 30°C. After 12 hours of reaction, 61.4 g of white solid (purity 96.8%) was obtained by filtration, and the product yield was 88.5%.
Embodiment 2
[0027] In a 1L four-neck flask, add 200ml of acetonitrile and 400ml of dichloromethane, weigh 60g of D-serine methyl ester hydrochloride (0.386mol) into the mixed solvent, stir evenly, cool to 0°C, and then slowly add SOCl 2 34.4ml (0.463mol), keep the temperature at 0℃, add SOCl 2 Then the temperature was raised to 30°C. After 12 hours of reaction, 63.4 g of white solid (purity 97%) was obtained by filtration, with a yield of 91.6%.
Embodiment 3
[0029] In a 1L four-neck flask, add 300ml of acetonitrile and 300ml of dichloromethane, weigh 60g of D-serine methyl ester hydrochloride (0.386mol) into the mixed solvent, stir well, cool to 0°C, and then slowly add SOCl 2 34.4ml (0.463mol), keep the temperature at 0℃, add SOCl 2 Then the temperature was raised to 20°C. After 12 hours of reaction, 64.92 g of white solid (purity 97%) was obtained by filtration, and the yield was 93.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com