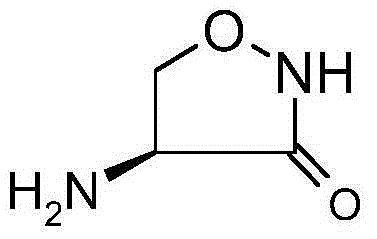
Patents
Literature
40 results about "D-cycloserine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An analogue of the amino acid D-alanine with broad-spectrum antibiotic and glycinergic activities. D-cycloserine interferes with bacterial cell wall synthesis by competitively inhibiting two enzymes, L-alanine racemase and D-alanine:D-alanine ligase, thereby impairing peptidoglycan formation necessary for bacterial cell wall synthesis. This agent may be bactericidal or bacteriostatic, depending on its concentration at the infection site and the susceptibility of the organism. In addition, D-cycloserine is an excitatory amino acid and partial agonist at the glycine binding site of the NMDA receptor in the central nervous system (CNS); binding to the central NMDA receptor may result in amelioration of neuropathic pain. Check for http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=469006&idtype=1 active clinical trials or http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=469006&idtype=1&closed=1 closed clinical trials using this agent. (http://nciterms.nci.nih.gov:80/NCIBrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&code=C47466 NCI Thesaurus)
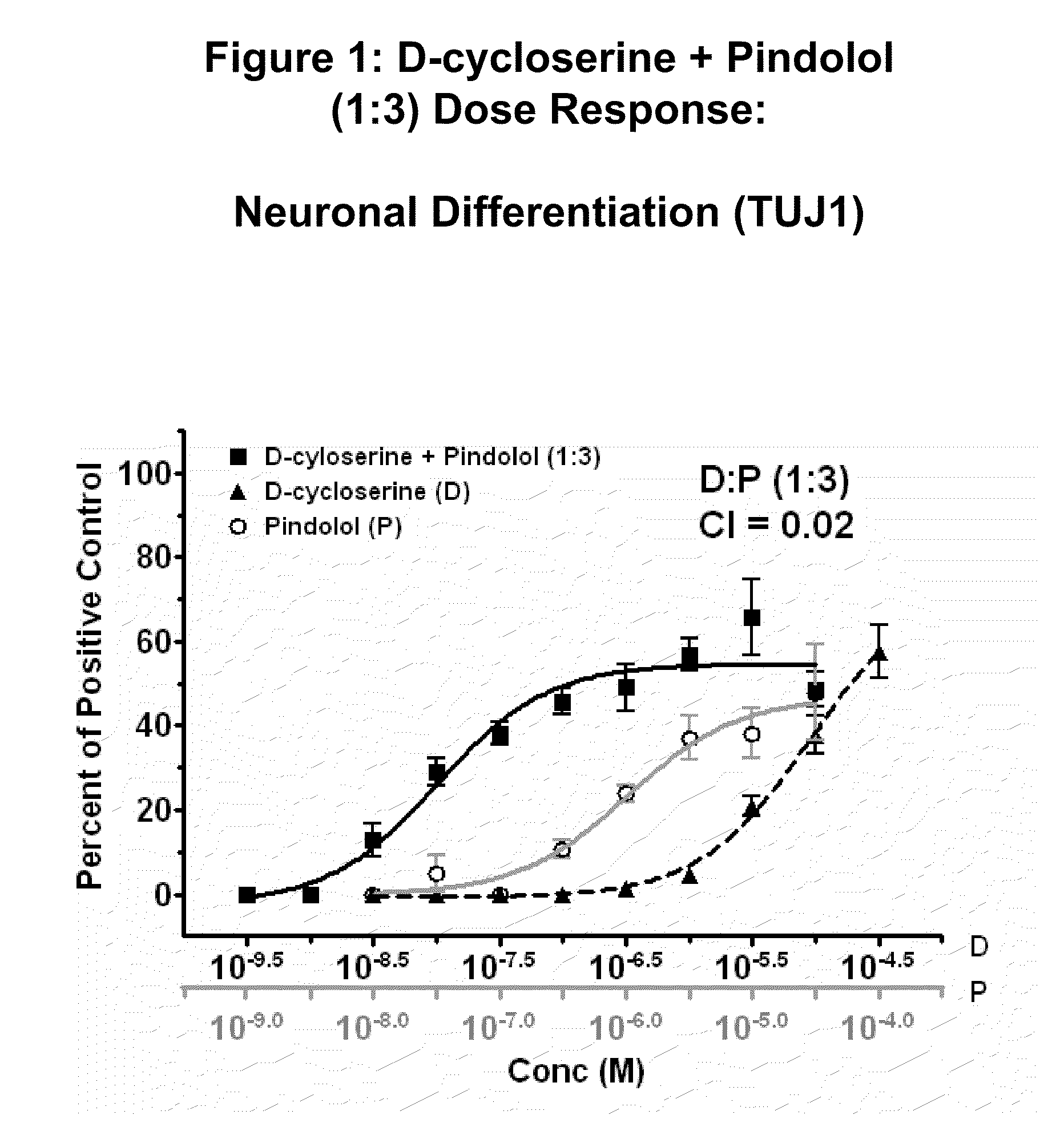
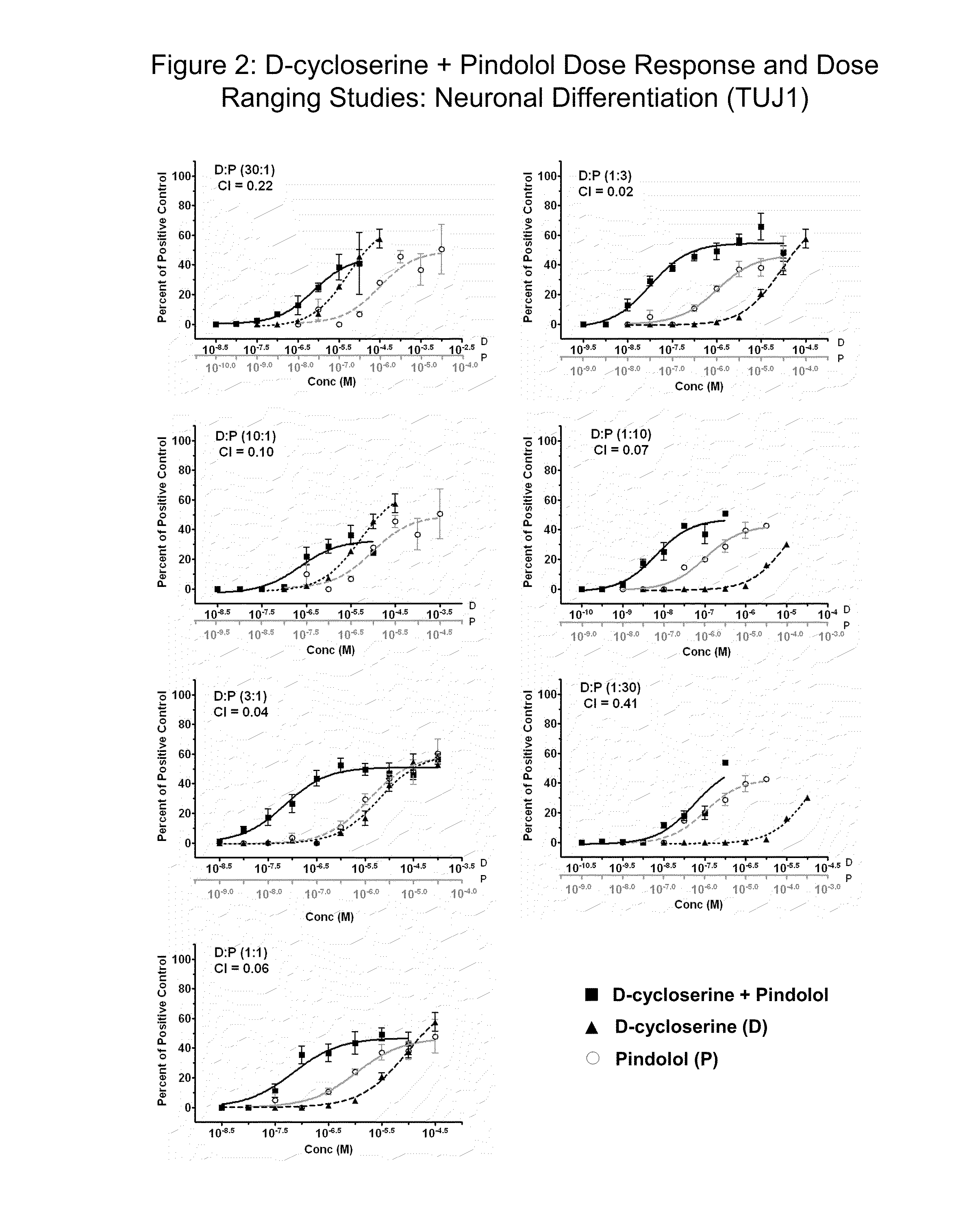
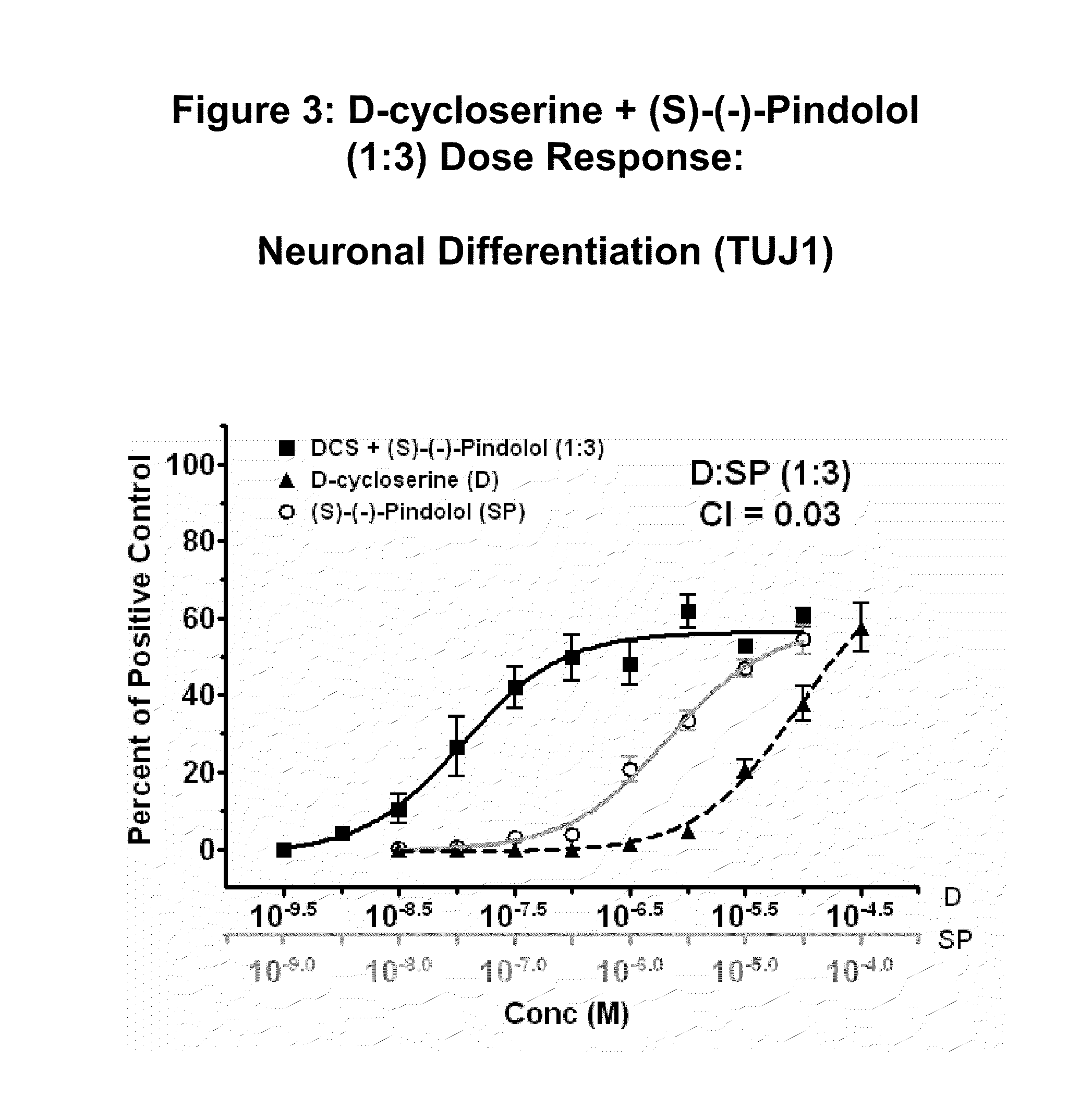
Modulation of neurogenesis using d-cycloserine combinations
InactiveUS20100216805A1Reduce decreaseLower Level RequirementsBiocideNervous disorderDiseaseNervous system
The disclosure provides compositions and methods for treating diseases and conditions of the central and peripheral nervous system by stimulating or increasing neurogenesis. The disclosure provides compositions and methods based on the use of D-cycloserine in combination with the neurogenic agent, which synergistically stimulates or activates the formation of new nerve cells.
Owner:BRAINCELLS INC
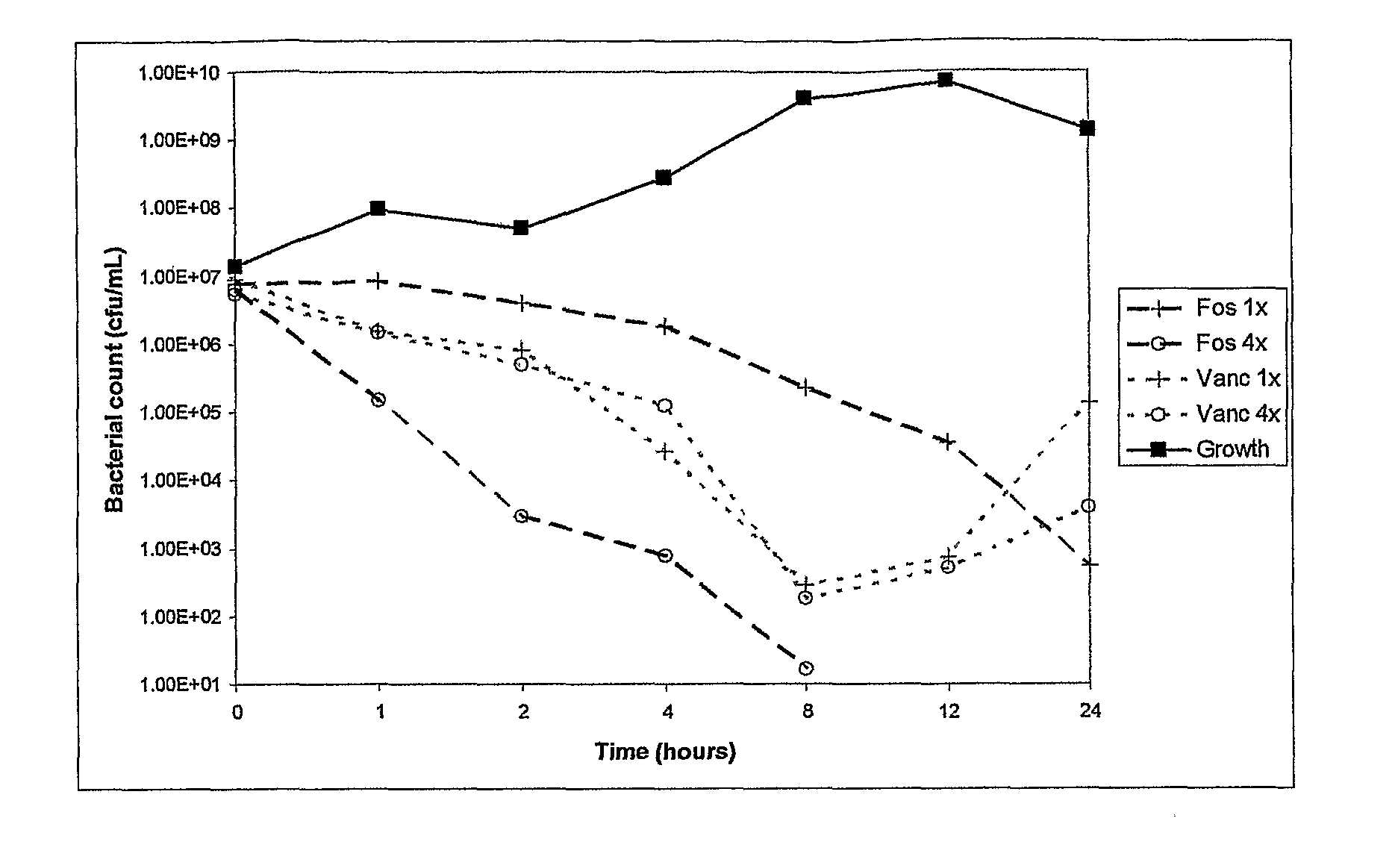
Antibacterial combination therapy for the treatment of gram positive bacterial infections
InactiveUS20110257078A1Reduce and eliminate contaminationAvoid developmentAntibacterial agentsBiocideBacteroidesMedicine
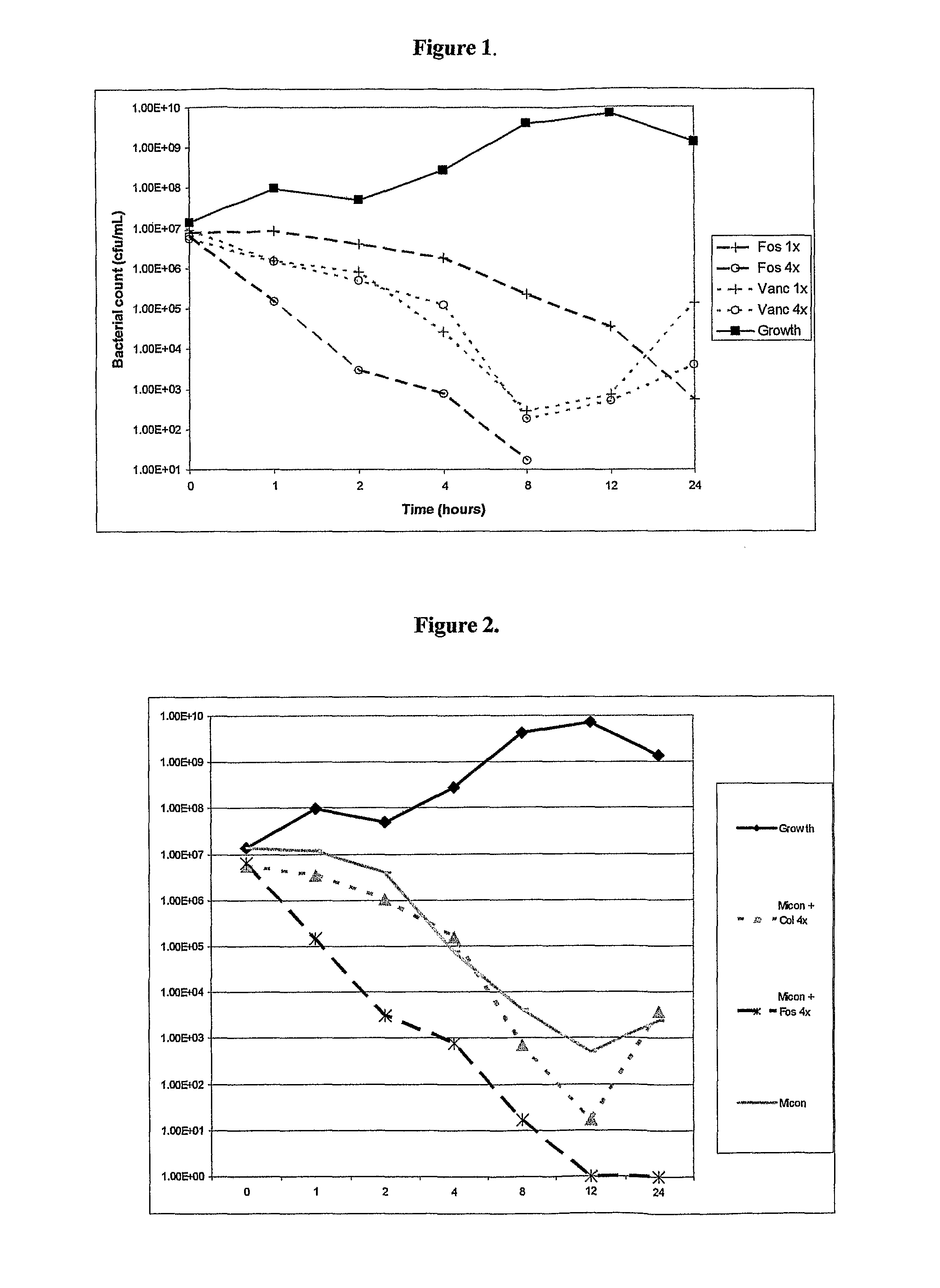
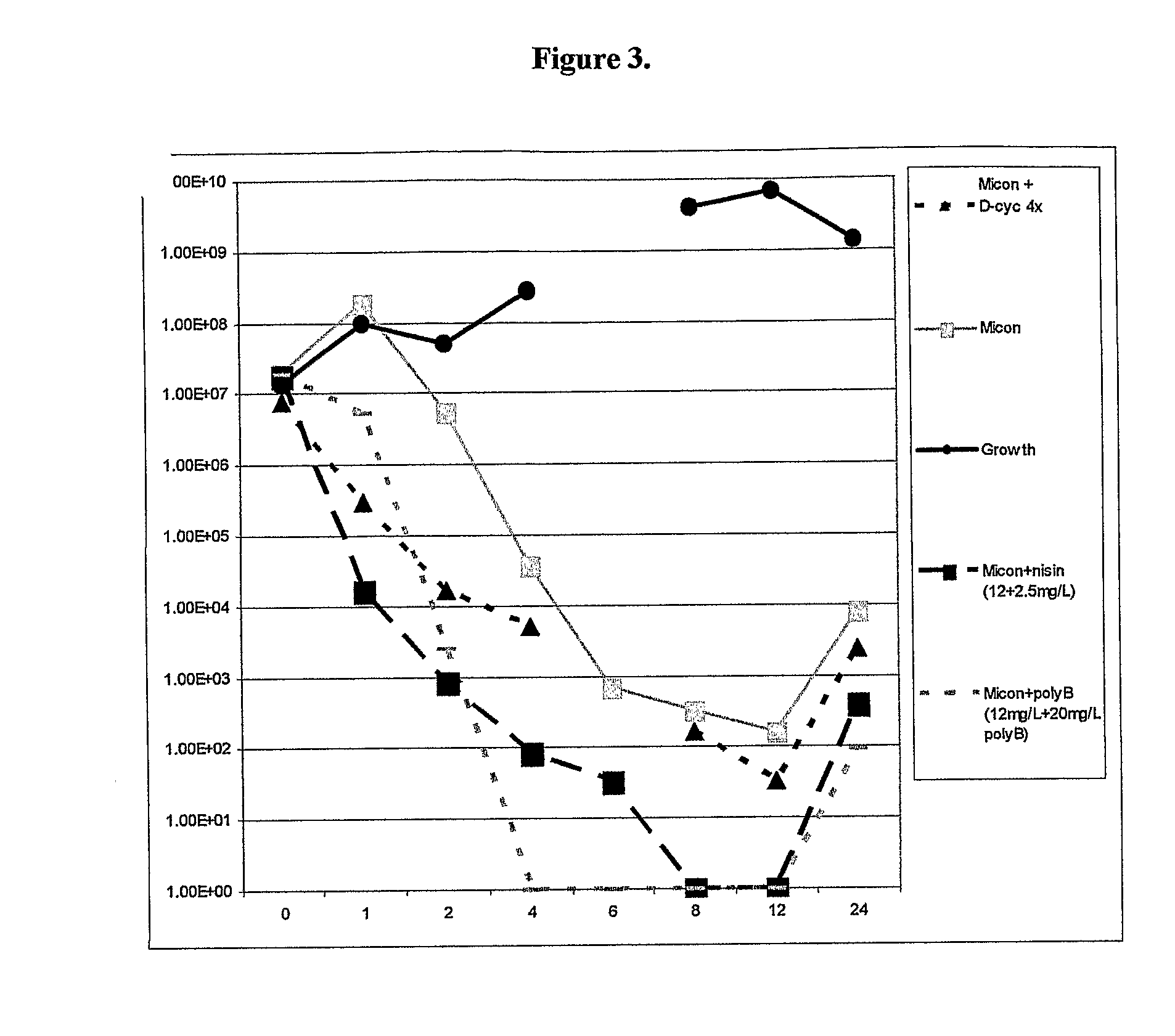
There is described a composition comprising a therapeutically active imidazole, and derivatives thereof, and an agent active on a bacterial cell surface selected from the group consisting of one or more of colistin, nisin, D-cycloserine, fosfomycin, fosfomycin trometamol, fosfomycin disodium and polymixin B, and derivatives thereof.
Owner:E THERAPEUTICS LTD
Methods for treating neuropsychiatric disorders
The invention provides methods for treating neuropsychiatric disorders such as schizophrenia, Alzheimer's Disease, autism, depression, benign forgetfulness, childhood learning disorders, closed head injury, and attention deficit disorder. The methods entail administering to a patient diagnosed as having a neuropsychiatric disorder a pharmaceutical composition containing (i) a therapeutically effective amount of D-alanine (or a modified form thereof), provided that the composition is substantially free of D-cycloserine, and / or (ii) D-serine (or a modified form thereof), and / or (iii) 105 to 500 mg of D-cycloserine (or a modified form thereof), and / or (iv) N-methylglycine (or a modified form thereof).
Owner:THE GENERAL HOSPITAL CORP
Method of Treating a Neurodegenerative Disorder
The present invention provides a method of treating a neurodegenerative disorder in a patient, comprising administration to the patient a therapeutically effective amount of D-cycloserine and its derivatives.
Owner:CHUNG SHAN MEDICAL UNIVERSITY +2
Method and compositions for treatment of chronic neuropathic pain
Chronic pain is treated in an individual suffering from chronic pain by administering to the individual an amount of a therapeutic containing a glycine receptor agonist such as D-cycloserine or a GlyT-1 glycine transporter antagonist such as sarcosine in an amount effective to treat the chronic pain. The therapeutic may also contain a secondary analgesic such as opiates, NSAIDs or cox-2 inhibitors. The analgesic can be formulated in a pharmaceutical composition in the form of an injectable solution that contains at least two different analgesics, at least one of the analgesics of which is a glycine receptor agonist or a GlyT-1 glycine transporter antagonist. Suitable pharmaceutical compositions contain D-cycloserine and / or sarcosine, optionally in combination with opiates, NSAIDs or cox-2 inhibitors.
Owner:APKARIAN TECH
Methods for treating neuropsychiatric disorders
InactiveUS20050250851A1High level of efficacyImprove usabilityBiocideNervous disorderAttention deficitsSerine
The invention provides methods for treating neuropsychiatric disorders such as schizophrenia, Alzheimer's Disease, autism, depression, benign forgetfulness, childhood learning disorders, closed head injury, and attention deficit disorder. The methods entail administering to a patient diagnosed as having a neuropsychiatric disorder a pharmaceutical composition containing (i) a therapeutically effective amount of D-alanine (or a modified form thereof), provided that the composition is substantially free of D-cycloserine, and / or (ii) D-serine (or a modified form thereof), and / or (iii) 105 to 500 mg of D-cycloserine (or a modified form thereof), and / or (iv) N-methylglycine (or a modified form thereof).
Owner:THE GENERAL HOSPITAL CORP
Mediucm for detecting Van A and Van B vancomycin-resistant entercocci and method of using the same
InactiveUS7364874B2Reduce detectionEasy to useBacteriaMicrobiological testing/measurementSodium lactateMicroorganism
Owner:TOKYO WOMENS MEDICAL UNIV
Enrichment culture medium
InactiveCN104845917AImprove separation rateReliable detectionBacteriaMicroorganism based processesYeast extractPyrone
The invention discloses an enrichment culture medium and belongs to the field of detection. The enrichment culture medium is characterized in that a formula contains beef liver powder, malt extract powder, yeast extract powder, sodium chloride, agar, ferric ammonium citrate, calcium lactate, D-seromycin, 2-ethoxymethylene-3,5-dihydroxy-y-pyrone, vitamin K1, sterile defiberized sheep blood and distilled water. Compared with the prior art, the enrichment culture medium plays roles in transporting and enriching.
Owner:钟学文
Remedies for spinocerebellar ataxia and compositions for treating spinocerebellar ataxia
InactiveUS7067545B1Reduce in quantityBiocideNervous disorderSpinocerebellar DegenerationsBULK ACTIVE INGREDIENT
Remedies for spinocerebellar degeneration or compositions for treating the same which contain as the active ingredient one or more members selected from among D-cycloserine, D-serine esters, D-serine and salts thereof. A method for treating spincerebellar degeneration which comprises administering to a patient with this disease in an efficacious dose of one or more members selected from among D-cycloserine, D-serine esters, D-serine and salts thereof.
Owner:MEIJI SEIKA KAISHA LTD
Methods for treating neuropsychiatric disorders
The invention provides methods for treating neuropsychiatric disorders such as schizophrenia, Alzheimer's Disease, autism, depression, benign forgetfulness, childhood learning disorders, closed head injury, and attention deficit disorder. The methods entail administering to a patient diagnosed as having a neuropsychiatric disorder a pharmaceutical composition containing (i) a therapeutically effective amount of D-alanine (or a modified form thereof), provided that the composition is substantially free of D-cycloserine, and / or (ii) D-serine (or a modified form thereof), and / or (iii) 105 to 500 mg of D-cycloserine (or a modified form thereof), and / or (iv) N-methylglycine (or a modified form thereof).
Owner:THE GENERAL HOSPITAL CORP
Sublingual Formulations of D-Cycloserine and Methods of Using Same
The invention provides methods and compositions for treating anxiety-related disorders in a subject. The methods include sublingually administering D-cycloserine to a subject with the anxiety-related disorder, either alone or in combination with extinction training.
Owner:MCDEVITT JASON P +2
Mediucm for detecting vana and vanb vancomycin-resistant enterocci and method of using the same
Van A and Van B vancomycin resistant enterococci detection media as well as a method of selectively detecting Van A and Van B vancomycin resistant enterococci clinically important in vancomycin resistant enterococci from testing microorganisms or specimens using the media. The media for selectively detecting Van A and Van B VRE from testing microorganisms and specimens are media where enterococci can grow where vancomycin, D-cycloserine and D-lactate are added. Preferably 32-256 mug / ml of vancomycin, 1-64 mug / ml of D-cycloserine, and 0.025-0.8 mol / l of sodium lactate are added to culture medium where enterococci can grow.
Owner:TOKYO WOMENS MEDICAL UNIV
Endostatin-like angiogenesis inhibition
InactiveUS20050014784A1Accelerate emissionsAvoid quenchingBiocideMaterial nanotechnologyAngiogenesis InhibitionEndostatin
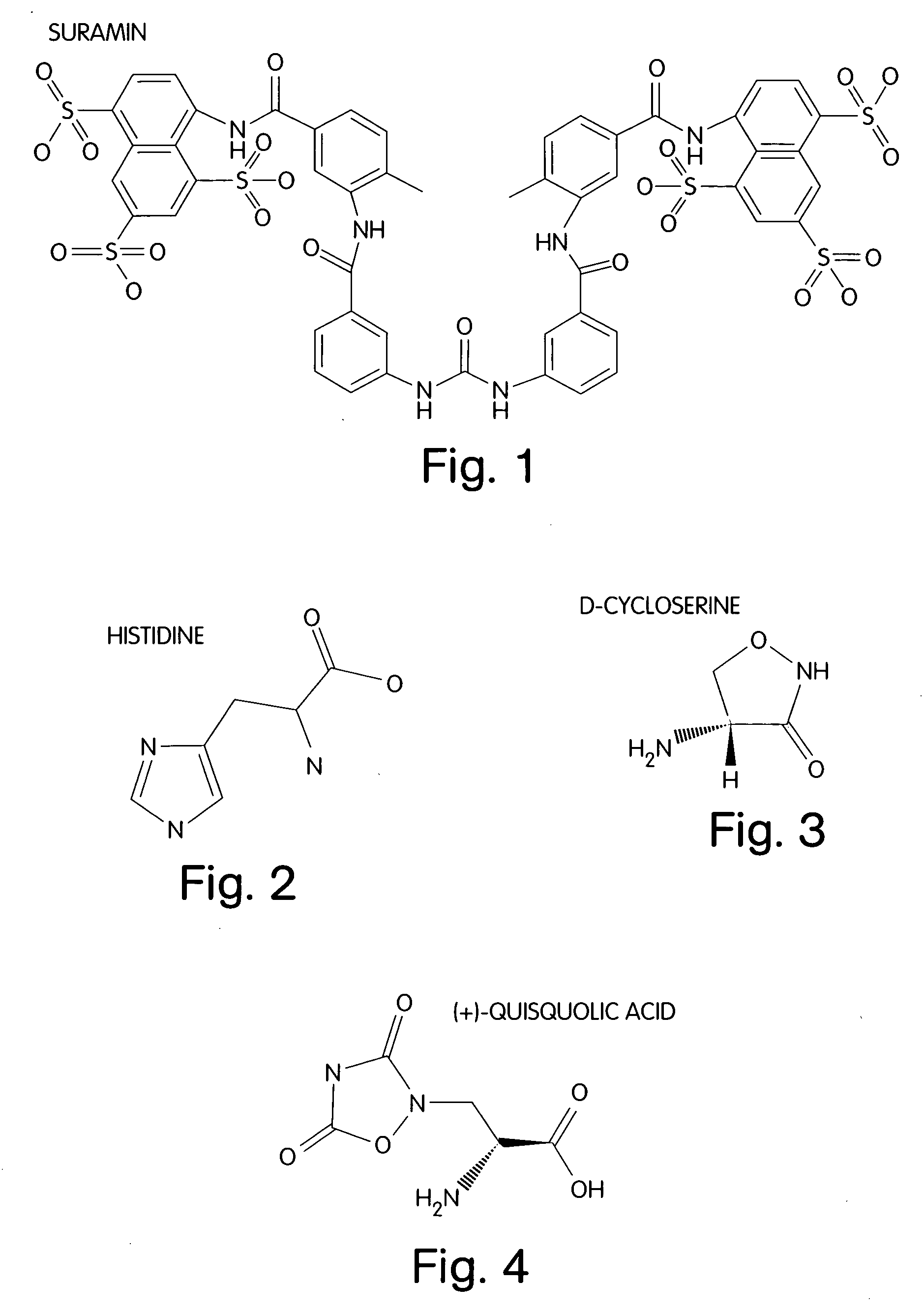
A treatment for cancer is provided. The treatment may include administering a therapeutic amount of L-histidine, D-cycloserine, quisqualic acid or suramin or analogs thereof.
Owner:MINERVA BIOTECH
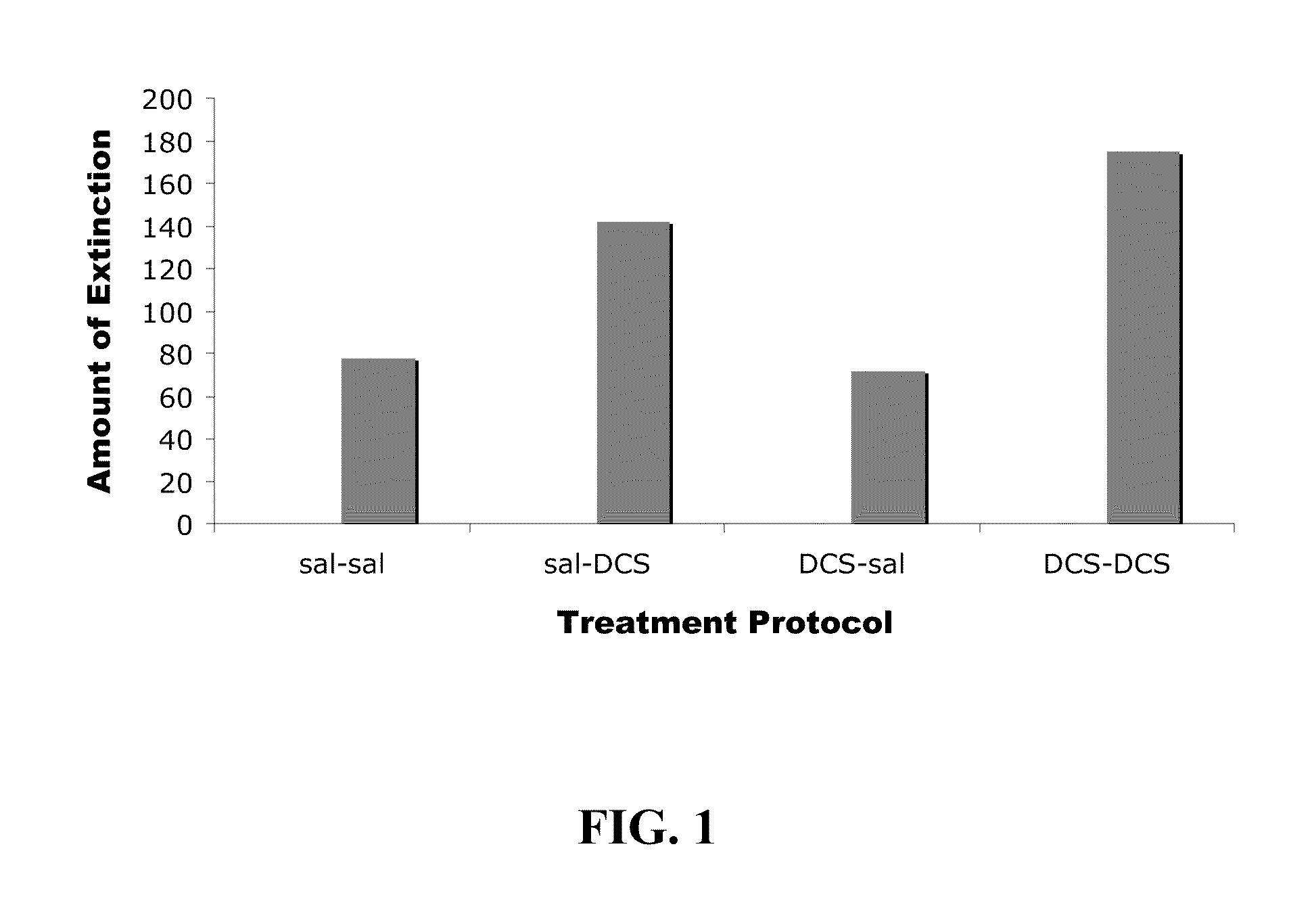
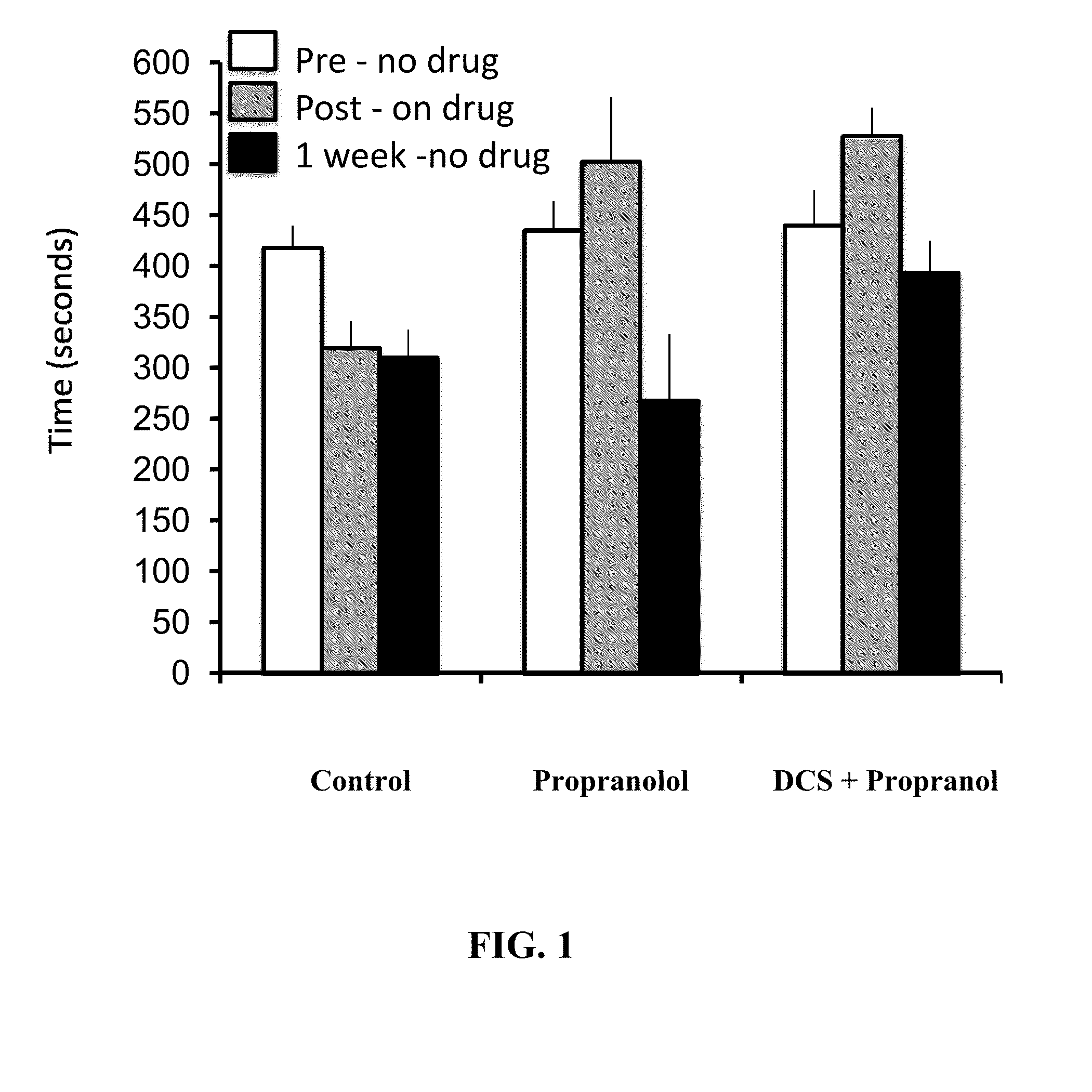
Method for facilitating extinction training using D-cycloserine
Methods are disclosed for improving treatment of various medical conditions via administration of D-cycloserine to facilitate extinction learning. Specifically, by administering D-cycloserine on a post-extinction training pre-sleep basis, subsequent to extinction training during the day, the methods can improve upon the known ability of D-cycloserine to facilitate extinction learning.
Owner:MCDEVITT JASON P +2
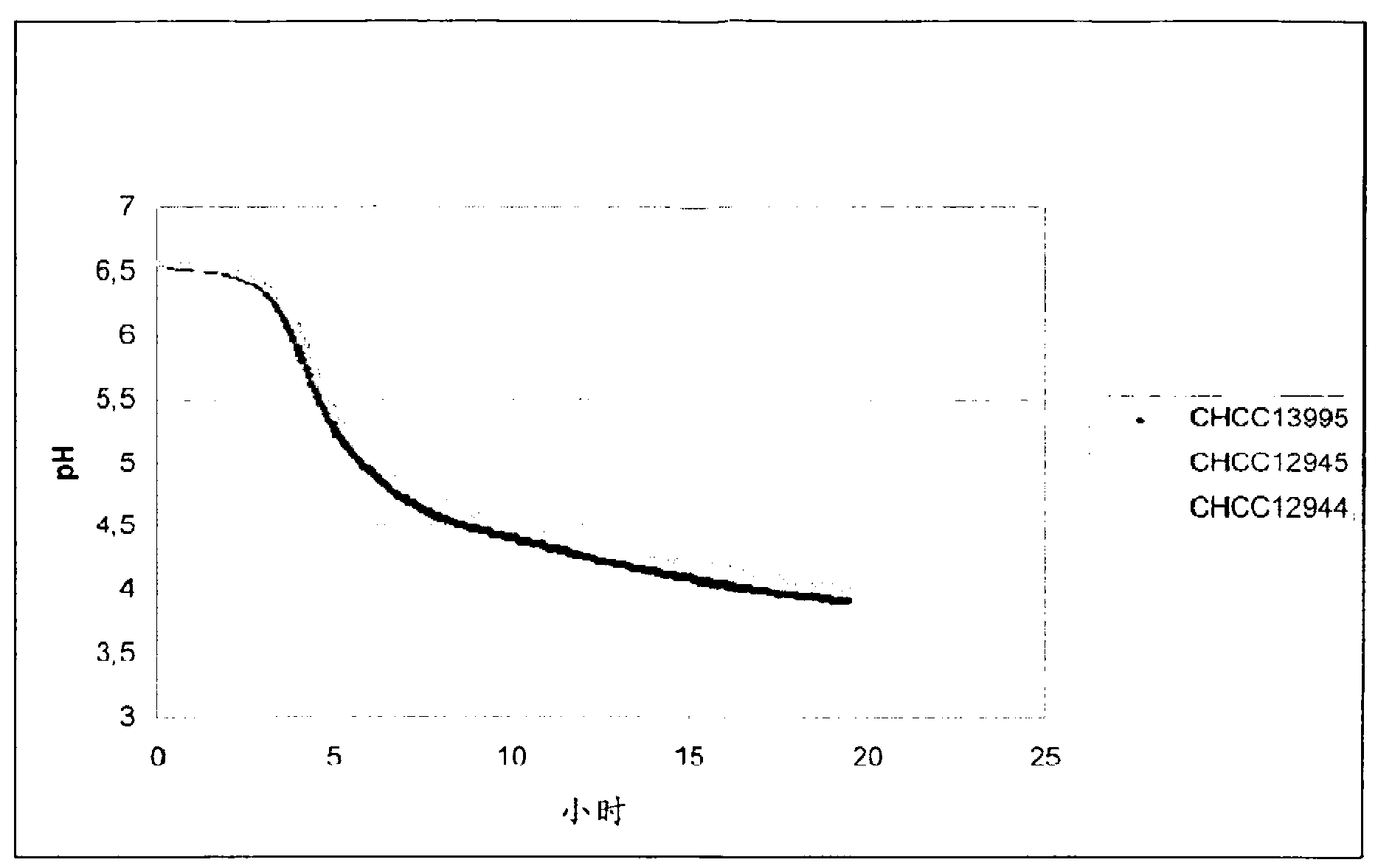
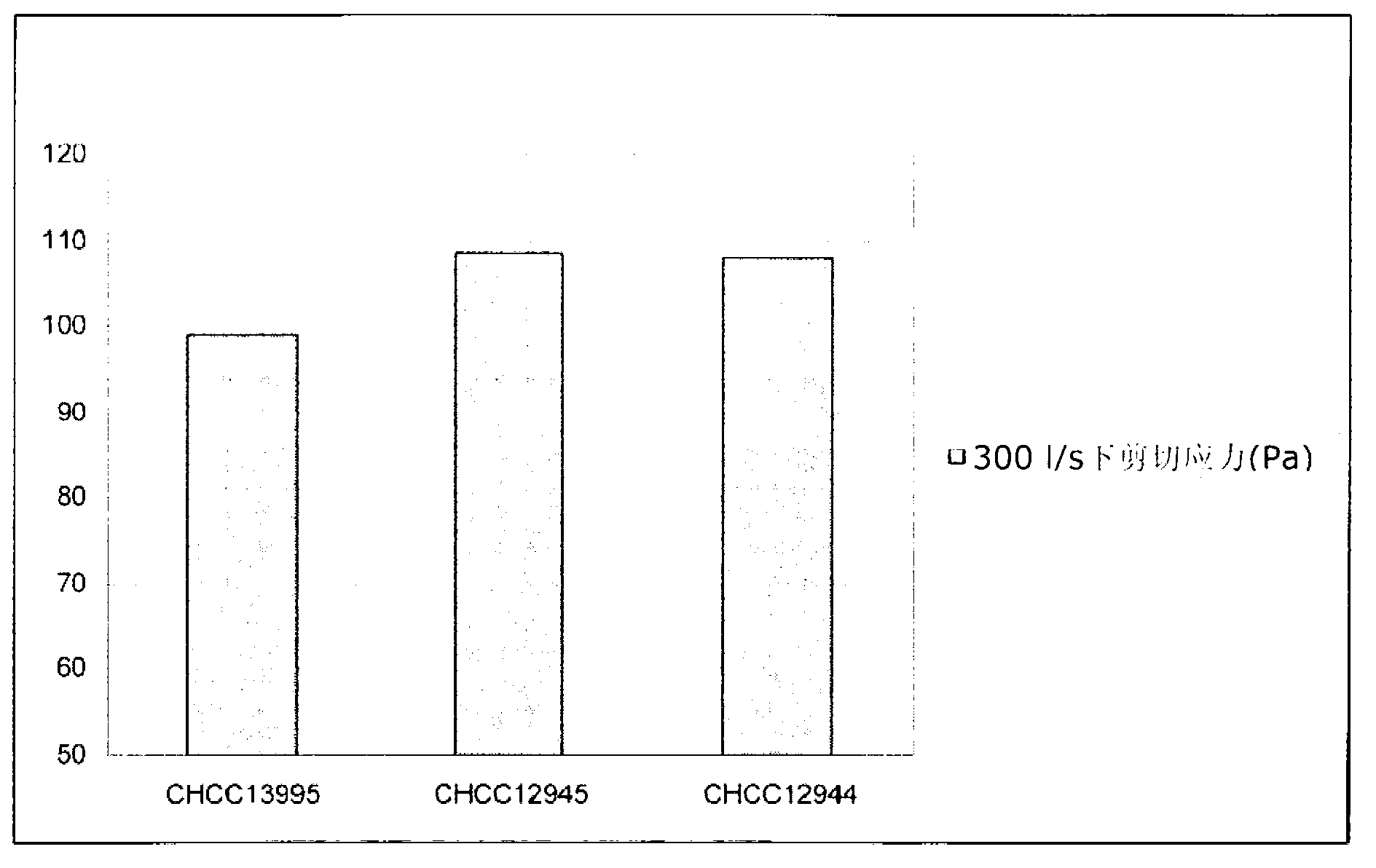
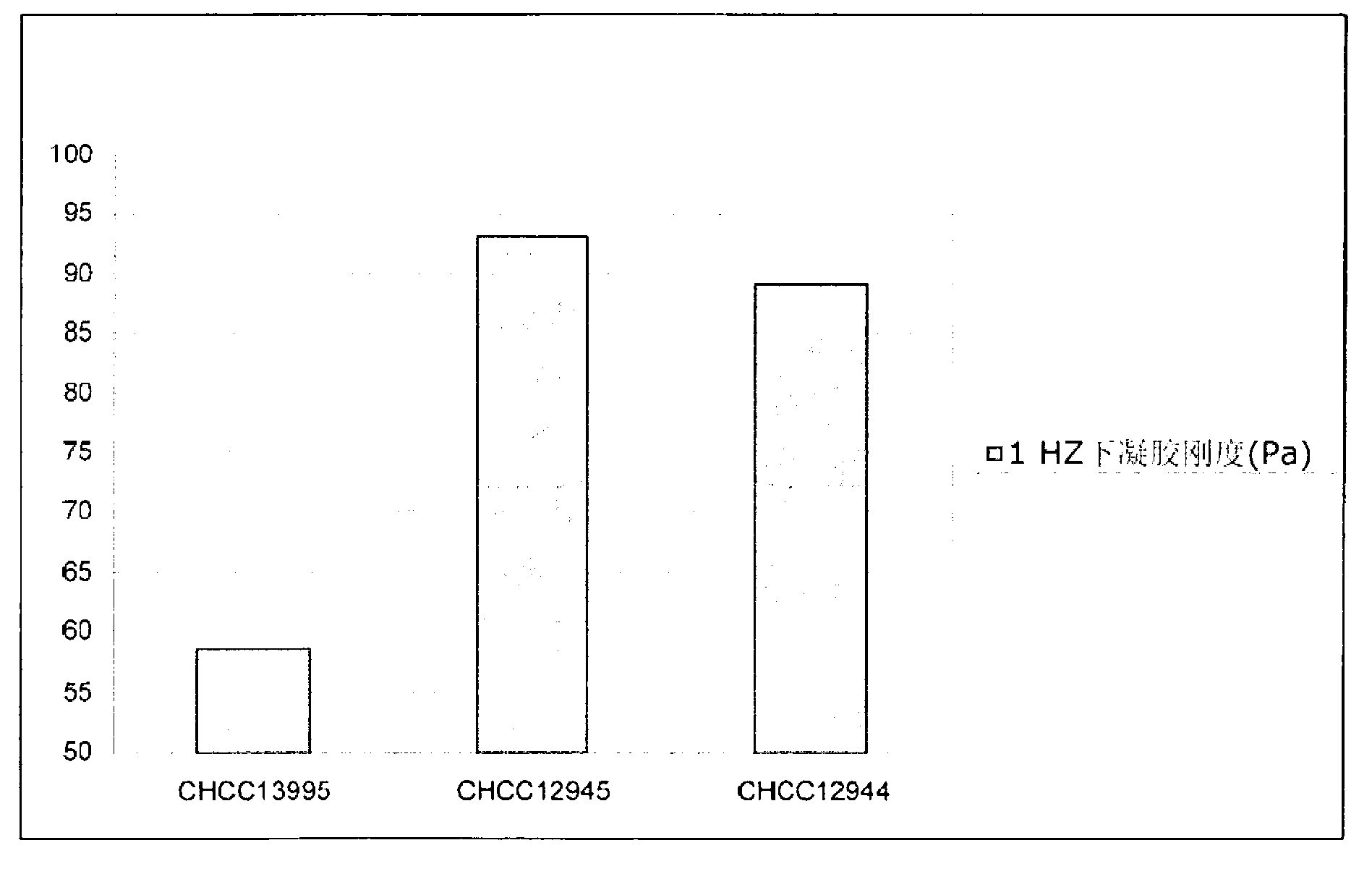
Texturizing lactic acid bacteria strains
The present invention relates to mutants of lactic acid bacteria which are resistant towards the antibiotic D-cycloserine and / or functionally equivalent antibiotics and which were found to give an increased texture when grown in milk while maintaining the other growth properties of the parent strain. The present invention, furthermore, relates to compositions comprising such mutants, and to dairy products fermented with the lactic acid bacteria resistant towards D-cycloserine and / or functionally equivalent antibiotics.
Owner:CHR HANSEN AS
Method for preparing D-cycloserine through one-pot method
InactiveCN110183391AAvoid splittingAtom economy is highOrganic chemistrySpecific rotationHydroxylamine Hydrochloride
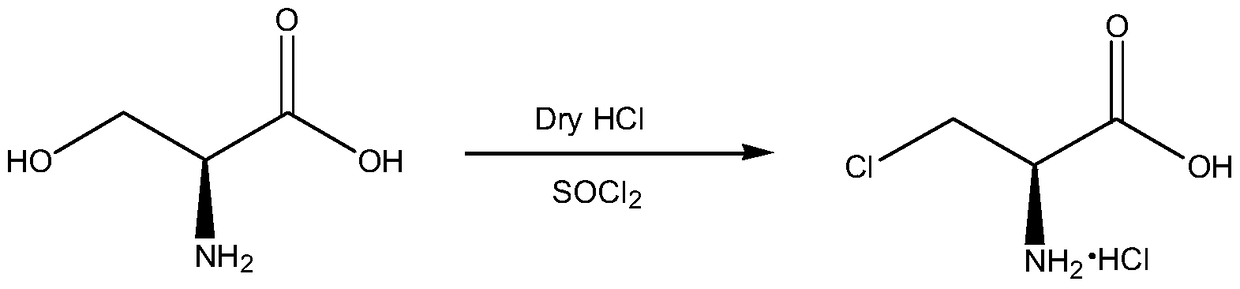
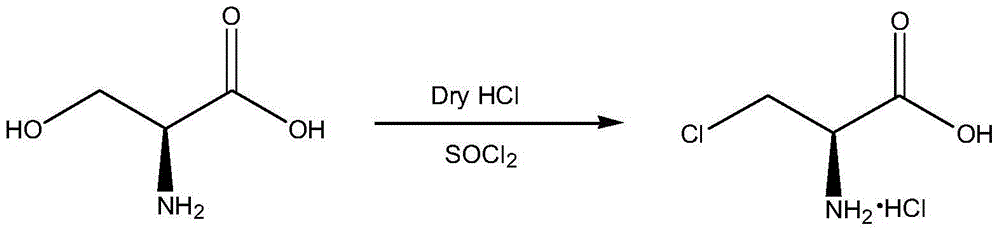
The invention discloses a method for preparing D-cycloserine through a one-pot method. The method comprises: carrying out a reaction on 3-chloro-D-serine and thionyl chloride in a solvent to obtain anintermediate 3-chloro-D-alanyl chloride hydrochloride solution; preparing a 35% sodium hydroxide aqueous solution, cooling to a temperature of less than 0 DEG C, controlling the temperature at -5 to5 DEG C, and adding the 3-chloro-D-alanyl chloride hydrochloride solution; uniformly stirring after completing the adding, controlling the temperature at -5 to 5 DEG C, and adding a hydroxylamine hydrochloride solution; slowly heating to a temperature of 20-30 DEG C after the adding, and adjusting the pH value of the solution to 10.5-12.0 by adding a 35% sodium hydroxide aqueous solution; carryingout standing liquid separation after completing the reaction; taking the water phase, and concentrating to achieve a near dry state; adding methanol, dissolving, and filtering to obtain a methanol solution; cooling to a temperature of -5 to 5 DEG C, and adjusting the pH value of the solution to 6.0-6.5 with a 60% acetic acid methanol solution; and carrying out stirring crystallization, carrying out centrifugation, drying the obtained wet product to obtain crude D-cycloserine, and refining to obtain the D-cycloserine finished product. According to the present invention, the product prepared bythe method has a liquid phase purity of more than 99.6% and a specific rotation of more than +110 DEG.
Owner:山东诚汇双达药业有限公司
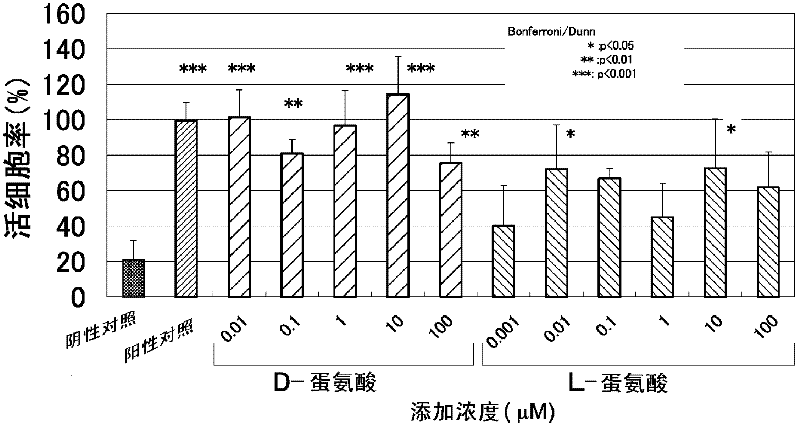
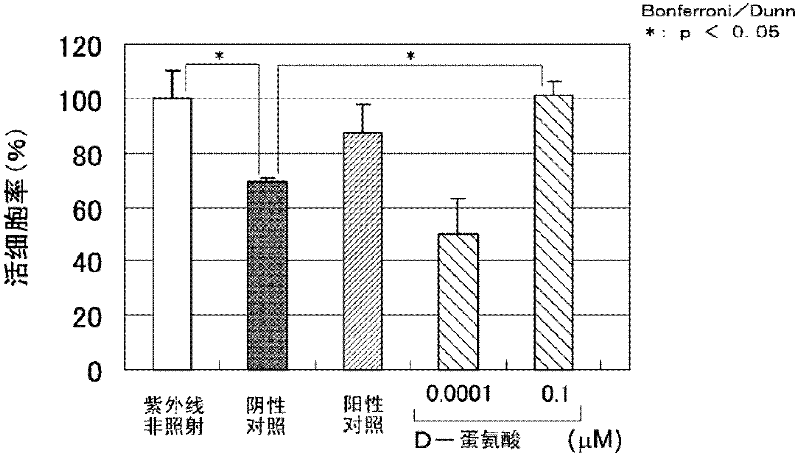
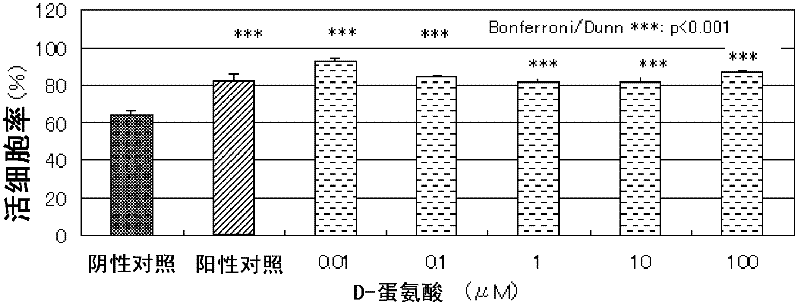
Composition for alleviating ultraviolet irradiation-induced damage
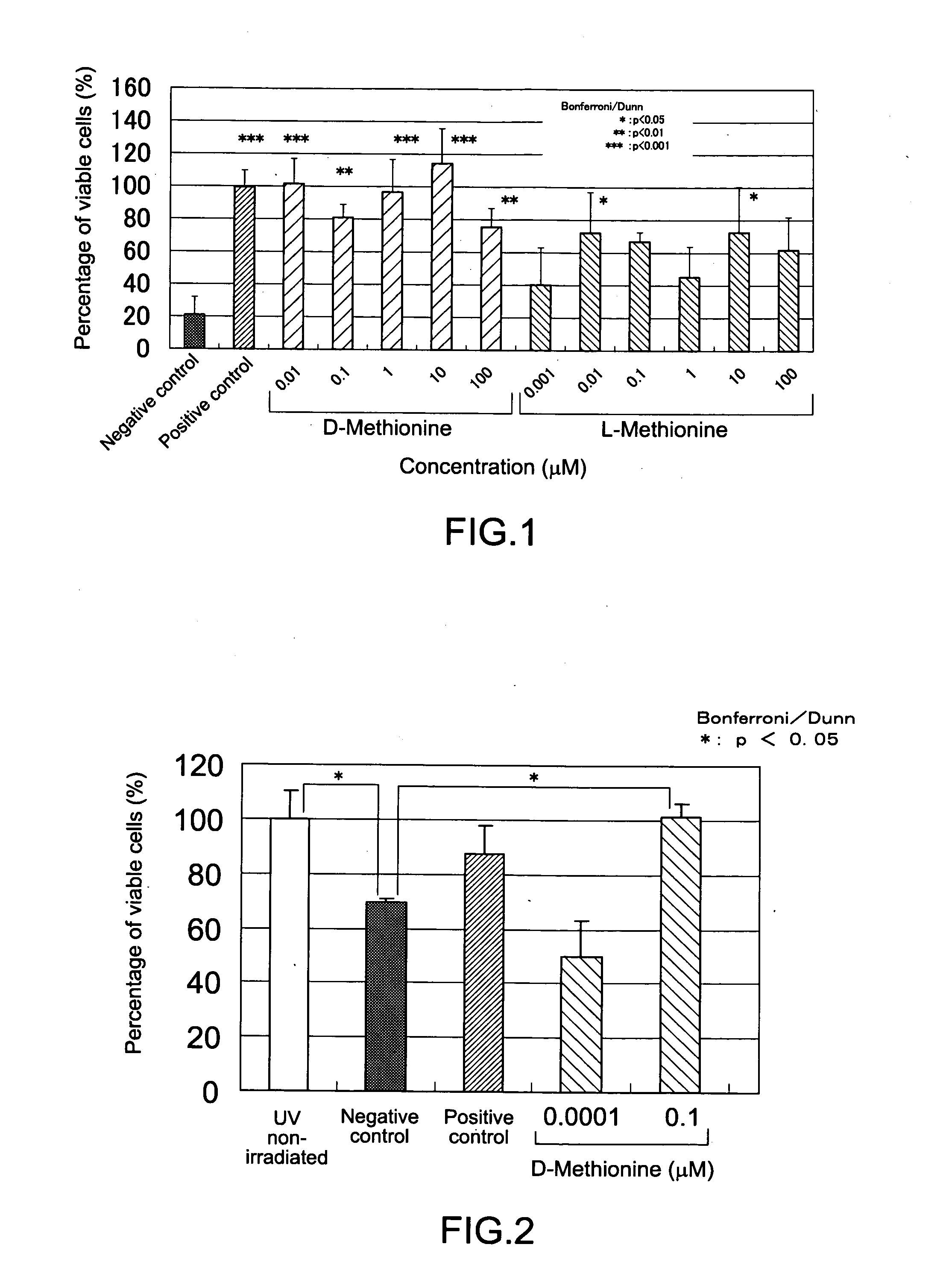
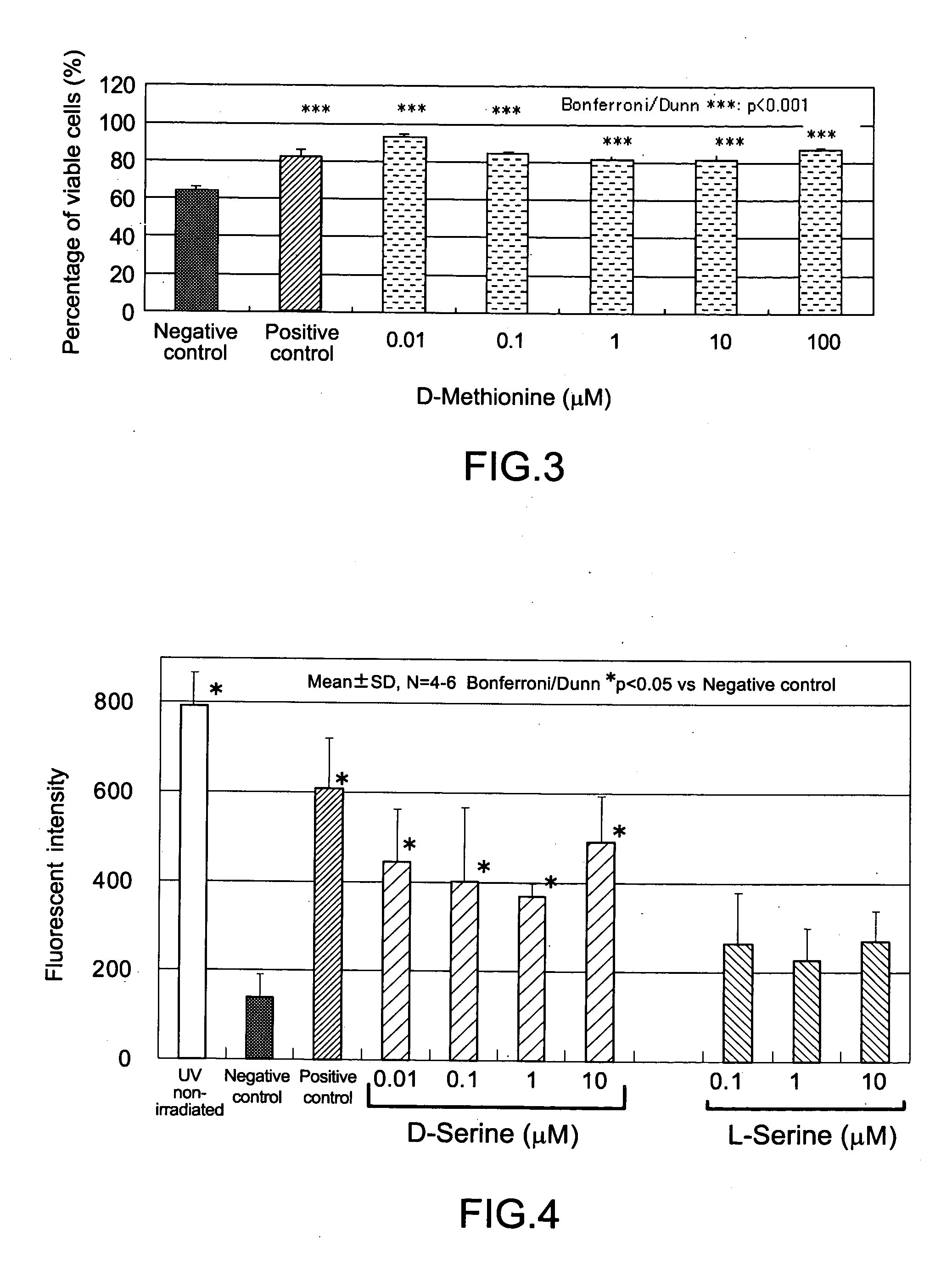
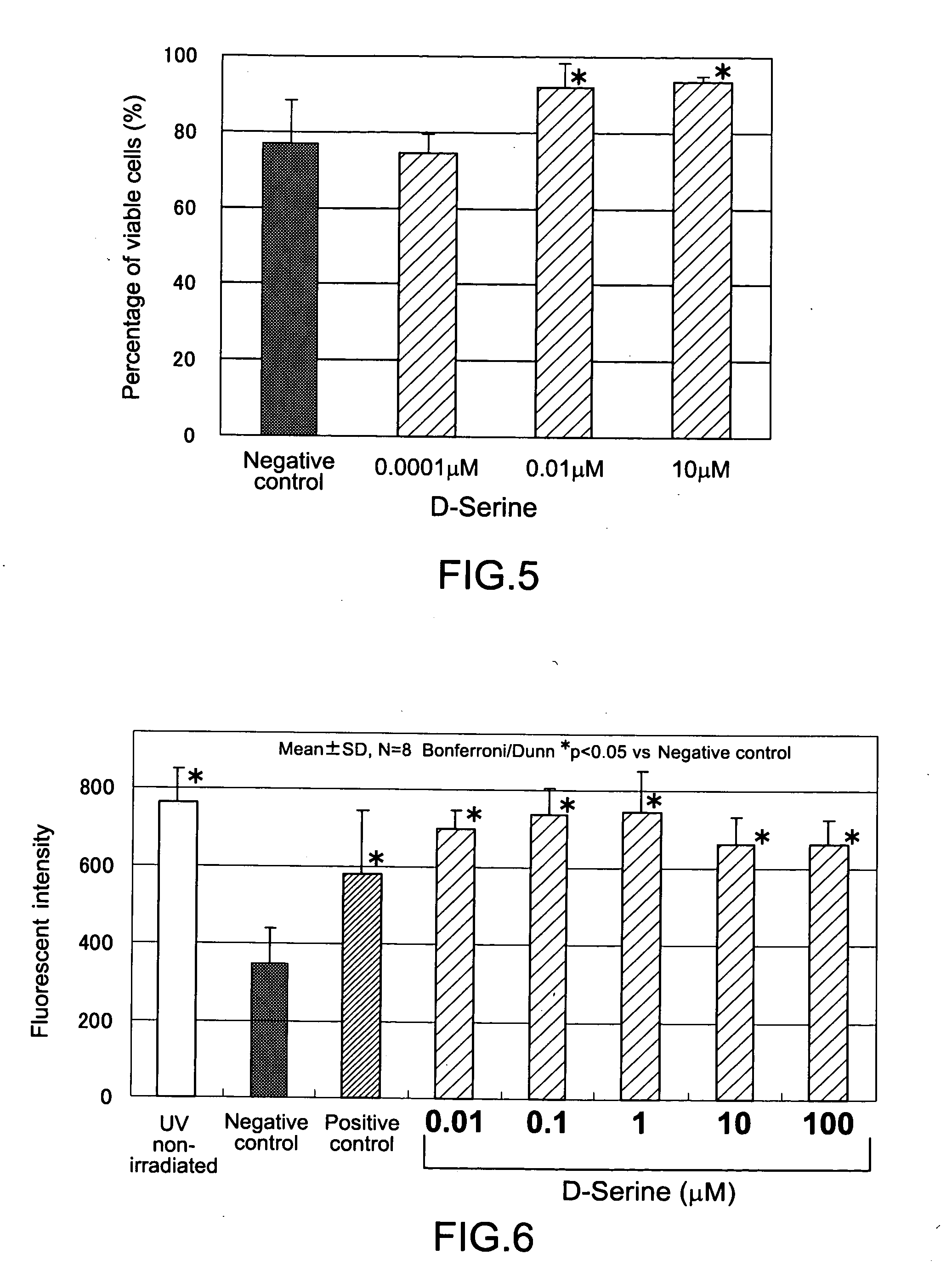
Disclosed are a composition for alleviating ultraviolet irradiation-induced damage, which is stable and safe and can be used on a daily basis, and a pharmaceutical composition, an external skin preparation, a food composition and a medicine for cataract, each comprising aforesaid composition for alleviating ultraviolet irradiation-induced damage. Specifically disclosed is a composition for alleviating ultraviolet irradiation-induced damage which contains one or more kinds of compounds selected from the group consisting of methionine, D-serine and derivatives and / or salts of the same. The aforesaid composition may be an external preparation for the skin, an anti-wrinkle agent, a sunscreen agent, a medicinal composition for treating and / or preventing skin diseases, a food composition or a pharmaceutical product for cataract. In the aforesaid composition for alleviating ultraviolet irradiation-induced damage, the aforesaid methionine may be D-methionine, and the aforesaid D-serine derivative may be D-cycloserine.
Owner:SHISEIDO CO LTD
Compositions and Methods to Improve Treatment of Medical Conditions Using D-Cycloserine
The invention describes methods and compositions for alleviating medical afflictions for which anxiety may cause or exacerbate the affliction. A subject suffering from the affliction is treated with a combination of a pharmaceutical compound that enhances learning, and a second pharmaceutical recognized to be useful for treatment of the affliction, wherein D-cycloserine is the pharmaceutical compound that enhances learning. Representative afflictions include pain, mood disorders, anxiety disorders including performance anxiety, insomnia, female sexual dysfunction, chronic fatigue, autism spectrum disorders, fibromyalgia, and attention deficit-hyperactivity disorder.
Owner:MCDEVITT JASON P +1
Method for treatment of chronic neuropathic pain
Chronic pain is treated in an individual suffering from chronic pain by administering to the individual an amount of a therapeutic containing a glycine receptor agonist such as D-cycloserine or a GlyT-1 glycine transporter antagonist such as sarcosine in an amount effective to treat the chronic pain. The therapeutic may also contain a secondary analgesic such as opiates, NSAIDs or cox-2 inhibitors. The analgesic can be formulated in a pharmaceutical composition in the form of an injectable solution that contains at least two different analgesics, at least one of the analgesics of which is a glycine receptor agonist or a GlyT-1 glycine transporter antagonist. Suitable pharmaceutical compositions contain D-cycloserine and / or sarcosine, optionally in combination with opiates, NSAIDs or cox-2 inhibitors.
Owner:APKARIAN TECH
Compositions and Methods to Improve Treatment of Medical Conditions Using D-Cycloserine
Owner:MCDEVITT JASON P +1
Composition for alleviating ultraviolet radiation-induced damage
Disclosed are a composition for alleviating ultraviolet radiation-induced damage, which is stable and safe and can be used on a day-to-day basis, and a medicinal composition, an external skin preparation, a food composition and a medicine for cataract, each comprising said composition for alleviating ultraviolet radiation-induced damage. Specifically disclosed is a composition for alleviating ultraviolet radiation-induced damage which contains one or more kinds of compounds selected from the group consisting of methionine, D-serine and derivatives and / or salts of the same. The aforesaid composition may be an external skin preparation, an anti-wrinkle agent, a sunscreen agent, a medicinal composition for treating and / or preventing skin diseases, a food composition or a medicine for cataract. In the aforesaid composition for alleviating ultraviolet radiation-induced damage, said methionine may be D-methionine, and said D-serine derivative may be D-cycloserine.
Owner:SHISEIDO CO LTD
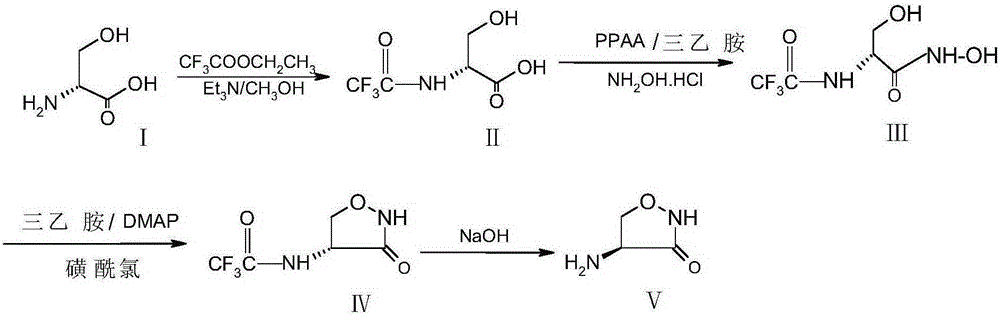
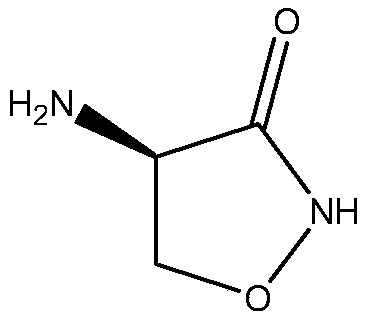
Preparation method of D-cycloserine
ActiveCN105198825AReduced racemizationHigh optical purityAntibacterial agentsOrganic chemistryCycloserineHydrolysis
The invention relates to a preparation method of D-cycloserine. The preparation method comprises steps of preparation of N-trifluoroacetyl-D-serine, preparation of (1-trifluoroacetylamino-2-hydroxyl) ethyl hydroxamate, preparation of D-4-trifluoroacetylamino-3-oxazolidinone and hydrolysis of D-4-trifluoroacetylamino-3-oxazolidinone. The preparation method has the benefits as follows: raw materials are easy to obtain, the reaction condition is mild, and the technological operation is simple; the racemization degree of D-cycloserine prepared with a synthetic route is reduced, the optical purity is improved, the product quality is stable, the follow-up three steps are performed with a 'one-pot' method, accordingly, emission of the three wastes and product loss are reduced, and the total yield of a reaction is improved.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
Method used for increasing 2,3-butylene glycol yield
ActiveCN103911398AIncrease profitIncrease concentrationMicroorganism based processesFermentationPenicillin2,3-Butanediol
The invention relates to a method used for increasing 2,3-butylene glycol yield. The method is mainly used for solving a problem of existing technology that yield of 2,3-butylene glycol is relatively low. According to the method, bacteria are subjected to high sugar resistance domestication firstly; the domesticated bacteria are used for fermentation culturing, and an antibiotic is added in fermentation culturing processes, wherein the antibiotic is at least one selected from penicillin, cephalosporin, bacillus, D-cycloserine, and the like which are capable of inhibiting cell wall synthesis, and polymyxin B, nystatin, amphotericin, or valinomycin which are capable of influencing cytomembrane functions, and adding concentration of the antibiotic ranges from 0.0001 to 5g / L. The method is capable of increasing 2,3-butylene glycol yield, and can be used for production of 2,3-butylene glycol.
Owner:SINOPEC SHANGHAI ENG +1
Medium for the isolation and enumeration of Clostridium butyricum
ActiveCN111763652BAchieve separationGrowth inhibitionBacteriaMicrobiological testing/measurementBacteroidesBromothymol blue
The invention relates to a culture medium for isolating and counting Clostridium butyricum, belonging to the field of microorganism separation and counting. Including tryptone 15.0g, beef extract powder 3.0g, yeast extract powder 1.0g, sodium chloride 5.0g, L-cysteine hydrochloride 0.5g, sodium thioglycolate 0.5g, xylose 10.0g, Bromothymol blue 0.04g, agar powder 15.0g, purified water 900ml, D-cycloserine 0.25g, kanamycin sulfate 0.012g, egg yolk 25ml. Compared with the prior art, the beneficial effects of the present invention are: first, the culture medium of the present invention can make Clostridium butyricum It can be clearly distinguished from other bacteria by visual observation, thereby achieving the purpose of isolating and counting Clostridium butyricum. Second, the effect is remarkable, direct and effective, and low in cost.
Owner:山东拓普生物工程有限公司
Culture medium enhancer suitable for isolating culture of brucella
InactiveCN105969688AEasy to separateHigh densityBacteriaMicroorganism based processesBacteroidesAdditive ingredient
A culture medium enhancer suitable for isolating culture of brucella is prepared from erythritol, glucose, animal serum, vitamins, amino acid, cell growth factors and the like. The content of ingredients in one liter of distilled water is present in the description, wherein the ingredients includes glucose, erythritol, tryptose, sodium chloride, horse serum, niacin, vitamins, vitamin B1, D-cycloserine, methylhexyl ether, polymyxin B, bacitracin, cycloheximide, nystatin, zinc sulfate, manganese sulfate, copper chloride, cobaltous sulfate, boric acid, ferric trichloride and aluminum trichloride. By using the culture medium enhancer suitable for isolating culture of brucella, growth of brucella in the culture medium can be effectively promoted while the growth of interference bacteria is suppressed. The culture medium enhancer is high in university oriented to the isolating culture of brucella, low in cost and convenient to prepare and use.
Owner:连云港市产品质量监督检验中心
Transport and enrichment culture medium
InactiveCN104846056AImprove separation rateReliable detectionMicrobiological testing/measurementMicroorganism based processesPhosphopeptideYeast extract
The invention discloses a transport and enrichment culture medium and belongs to the field of detection. The transport and enrichment culture medium is characterized in that a formula contains beef liver powder, malt extract powder, yeast extract powder, sodium chloride, agar, ferric ammonium citrate, calcium lactate, D-seromycin, 2-ethoxymethylene-3,5-dihydroxy-y-pyrone, vitamin K1, casein phosphopeptides, sterile defiberized sheep blood and distilled water. Compared with the prior art, the transport and enrichment culture medium plays roles in transporting and enriching.
Owner:李本传
Method for treating dementia
ActiveUS20160235719A1Easy to learnEnhance memoryHeterocyclic compound active ingredientsSide effectCycloserine
Methods are disclosed for treating dementia, including mild cognitive impairment, via administration of D-cycloserine, or a prodrug thereof, on a tolerance-inhibiting basis. Specifically, by administering D-cycloserine on a tolerance-inhibiting basis, tolerance to D-cycloserine is less likely than would occur via daily administration, enhancing benefits and reducing costs and side effects. Pharmaceutical compositions useful for the treatment of dementia are additionally disclosed.
Owner:COLLEGE OF WILLIAM & MARY
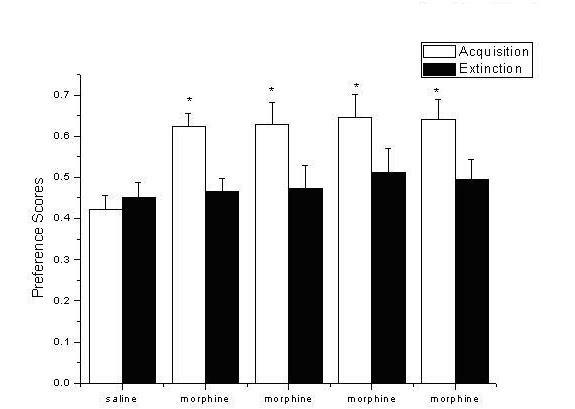
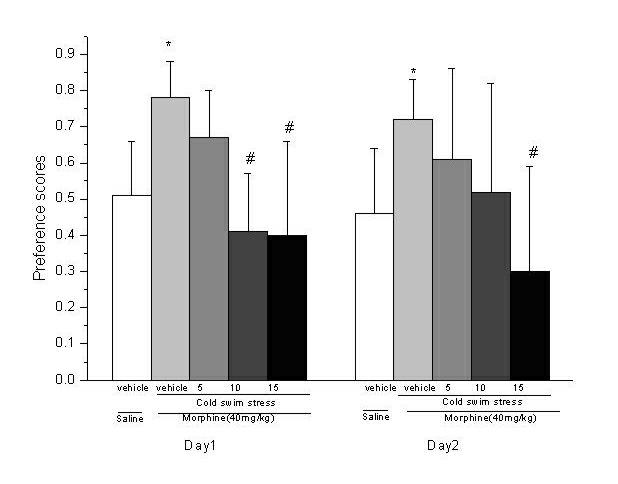
Application of D-seromycin in preparing medicine for treating Morphine psychological dependence behavior caused by stress
InactiveCN102579436ALittle side effectsDoes not affect physiological activityNervous disorderHeterocyclic compound active ingredientsNR1 NMDA receptorHuman body
The invention relates to an application of D-seromycin in preparing medicine for treating Morphine psychological dependence behavior caused by stress, which belongs to the medicine technical field. A glutamic acid system in brain plays an important role in drug addiction process, and N-methyl-D-aspartic acid (NMDA) receptor which can be combined with glutamic acid to play a physiological effect exists in central nervous system. The NMDA receptor can be functionally inhibited by acting on different recognition sites. At present more side effects are brought by completely inhibiting the NMDA receptor, so antagonist which not only can block the pathological activation of the NMDA receptor but also is free from influencing the physiological activity of the NMDA receptor is important to discover. DCS is a serine natural derivative and is combined with a glycine site in NMDA receptor NR1 subunit to play an effect of excitant, so that internal flow of calcium ions in cells can be increased, but no neurotoxicity is produced. Since the prepared medicine not only can partially excite the NMDA receptor, but also is free from influencing the physiological activity, the medicine has small side effect on the human body.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI +1
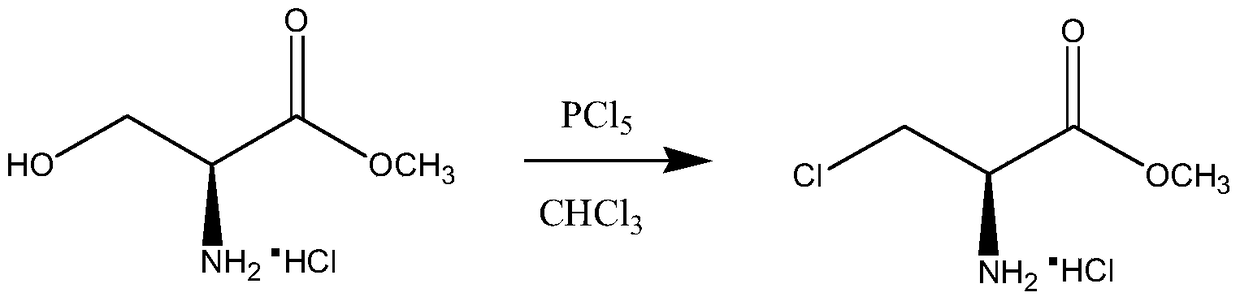
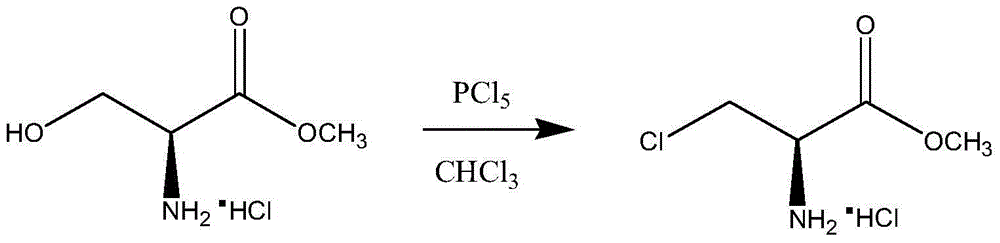
A kind of synthetic method of d-cycloserine intermediate
ActiveCN106146327BImprove operational safetyHigh yieldOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterOrganic solvent
Disclosed is a method of synthesizing a 3-halo-D-alanine methyl ester or an acid salt thereof. By reacting a D-serine methyl ester or an acid salt thereof and a halogenation reagent in an organic solvent, the method prepares a 3-halo-D-alanine methyl ester or an acid salt thereof. The compound is an important intermediate for preparing a D-cycloserine. The method and process employed in the present invention are easy to operate and highly safe, require a moderate reaction condition, have a high yield, and are low in cost, and reduce waste acid generation considerably and are suitable for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Synthetic method of D-cycloserine intermediate
ActiveCN106146327AImprove operational safetyHigh yieldOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterOrganic solvent
The invention relates to a synthetic method of a D-cycloserine intermediate. According to the method, 3-halo-D-alanine methyl ester or an acid salt of 3-halo-D-alanine methyl ester is prepared from D-serine methyl ester or an acid salt of D-serine methyl ester and a halogenating agent through reaction in an organic solvent, and is an important intermediate for preparing D-cycloserine. The synthetic method adopts a process simple and convenient to operate, is high in safety, mild in reaction condition, high in yield and low in cost, greatly reduces production of waste acid and is quite suitable for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com