A kind of preparation method of chiral four-membered ring taxane side chain compound
A compound and four-membered ring technology, which is applied in the field of preparation of chiral four-membered ring taxane side chain compounds, can solve the problems of unfavorable market promotion, high price, and high cost of the four-membered ring taxane side chain, and can reach the price The effect of low cost, simple operation and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
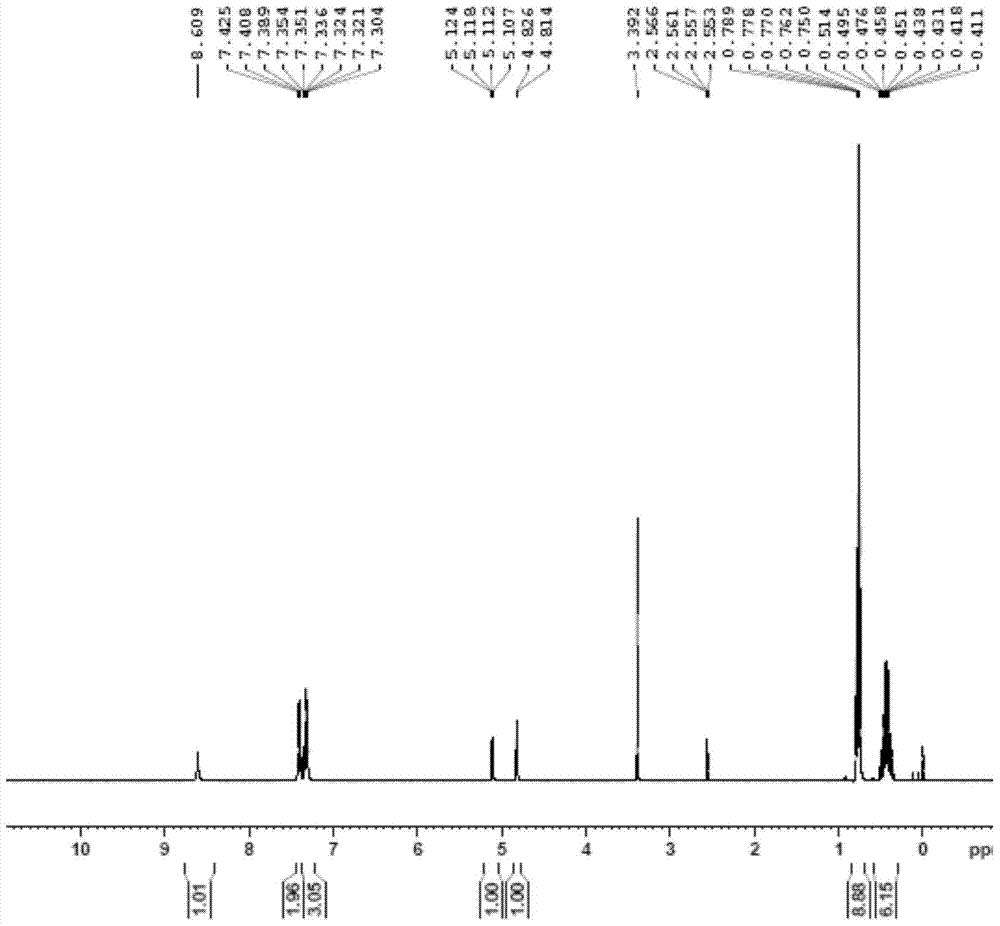
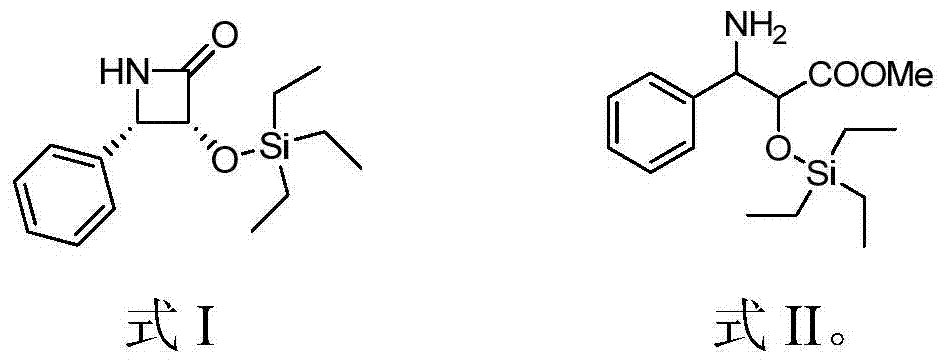
[0021] Add (-)-cytisine (2mmol) into tetrahydrofuran (20mL), cool to -30°C, add butyllithium (2mmol), then add the compound represented by formula II (2mmol), and react for 1 hour , TLC showed that the reaction of the raw materials was complete, the reaction solution was poured into a saturated aqueous ammonium chloride solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain a crude product. The crude product is purified by petroleum ether / ethyl acetate silica gel column chromatography, and the yield is 34%. figure 1 shown.
[0022] The control of the reaction temperature of the present embodiment is accomplished under the protection of nitrogen.
[0023] 1H-NMR (400MHz, d6-DMSO): 0.44 (m, 6H); 0.76 (t, J = 7.9, 9H); 4.79 (d, J = 4.7, 1H); 5.08 (dd, J = 4.7, J = 2.7,1H); 6.17(br,s,1H); 7.28-7.38(m,5H)
[0024]
Embodiment 2
[0026] Add (-)-cytisine (10mmol) into tetrahydrofuran (20mL), at 25°C, after adding butyl lithium (10mmol), add the compound shown in formula II (2mmol), and react for 1 hour, TLC shows After the reaction of the raw materials was complete, the reaction solution was poured into a saturated aqueous ammonium chloride solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain a crude product. Crude product obtains refined product with sherwood oil / ethyl acetate silica gel column chromatography, and yield is 45%, and the obtained product, i.e. the chiral four-membered ring taxane side chain compound shown in formula I, the proton nuclear magnetic resonance spectrum as shown in figure 1 shown.
[0027] The control of the reaction temperature of the present embodiment is accomplished under the protection of nitrogen.
[0028]
Embodiment 3
[0030] Add (-)-cytisine (3mmol) to tetrahydrofuran (20mL), put it in an ice-water bath, cool to 0°C, add butyllithium (3mmol), and then add the compound shown in formula II (2mmol) , After reacting for 1 hour, TLC showed that the reaction of the raw materials was complete. The reaction solution was poured into a saturated ammonium chloride aqueous solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain a crude product. The crude product is purified by petroleum ether / ethyl acetate silica gel column chromatography, and the yield is 92%. figure 1 shown.
[0031] The control of the reaction temperature of the present embodiment is accomplished under the protection of nitrogen.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com