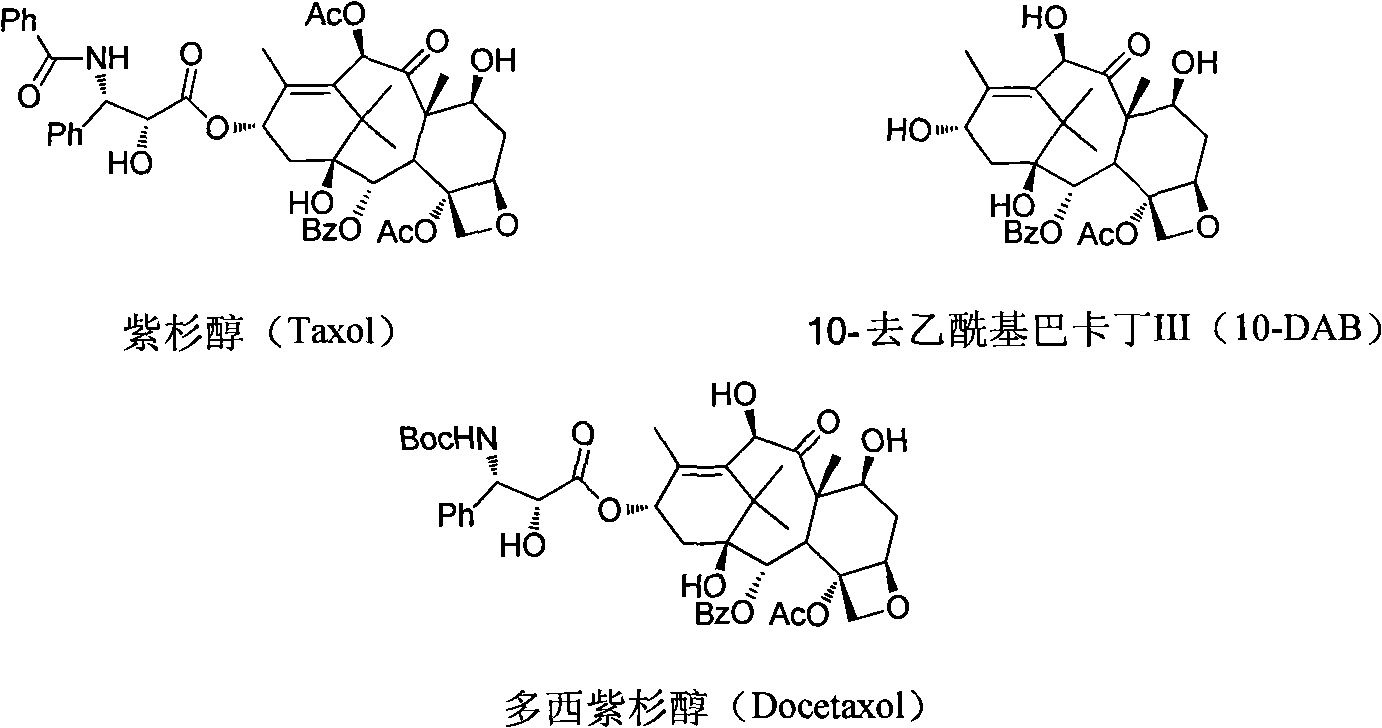
Preparation method of new taxane derivative
A taxane and derivative technology, applied in the field of docetaxel, can solve the problems of unstable intermediates, harsh conditions, many steps, etc., and achieve the effects of overcoming many reaction steps and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
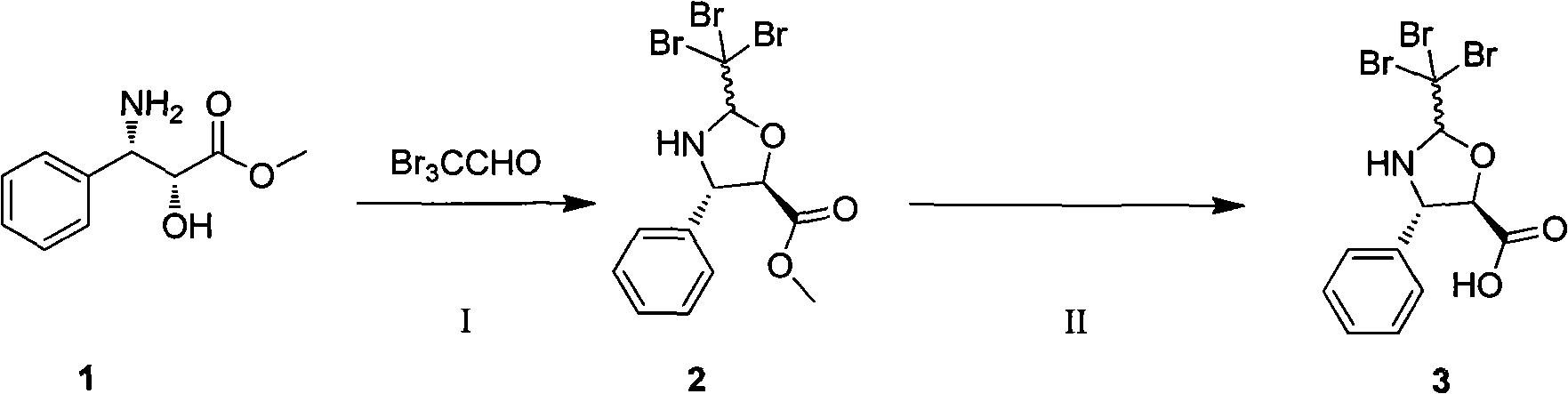
[0026]
[0027] In a 25 mL round bottom flask, (2R,3S)-3-phenylisoserine methyl ester (1.0 g, 5.1 mmol) was dissolved in toluene, tribromoacetaldehyde (1.57 g, 5.6 mmol) and a catalytic amount of Pyridinium p-toluenesulfonate, oil bath 120 ° C, stirred for 2 hours, TLC detection, raw materials disappeared, stop the reaction, remove toluene under reduced pressure, column chromatography purification (petroleum ether: ethyl acetate = 5: 1) to obtain product 2 : 2.29g, yield 98%.
[0028] (4S,5R)-2-Trichloromethyl-4-phenyl-5-carboxy-1,3-oxazolane: ESI-MS m / z: 455.8454[M+H] + ; 1 H-NMR (400MHz, CDCl 3 ): δ7.52 (d, 2H), 7.51-7.32 (m, 3H), 5.47 (d, 1H), 4.71 (s, 1H), 4.70 (d, 1H), 3.76 (s, 3H).
Embodiment 2
[0030]
[0031]Dissolve compound 2 (457.9mg, 1.0mmol) in 4.5mL ethanol-water (8:1), add lithium hydroxide (71.8mg, 3.0mmol), stir at room temperature for 3h, remove the methanol solvent under reduced pressure, acidify with 2N hydrochloric acid , extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain compound 3: 403.9 mg, yield 91%.
[0032] (4S,5R)-2-Trichloromethyl-4-phenyl-5-carboxy-1,3-oxazolane: ESI-MS m / z: 441.8309[M+H] + ; 1 H-NMR (400MHz, DMSO): δ7.60(d, 2H), 7.36-7.25(m, 3H), 5.42(s, 1H), 5.39(s, 1H), 4.94-4.90(m, 1H), 4.63-4.59 (m, 2H), 4.34 (d, 1H).
Embodiment 3
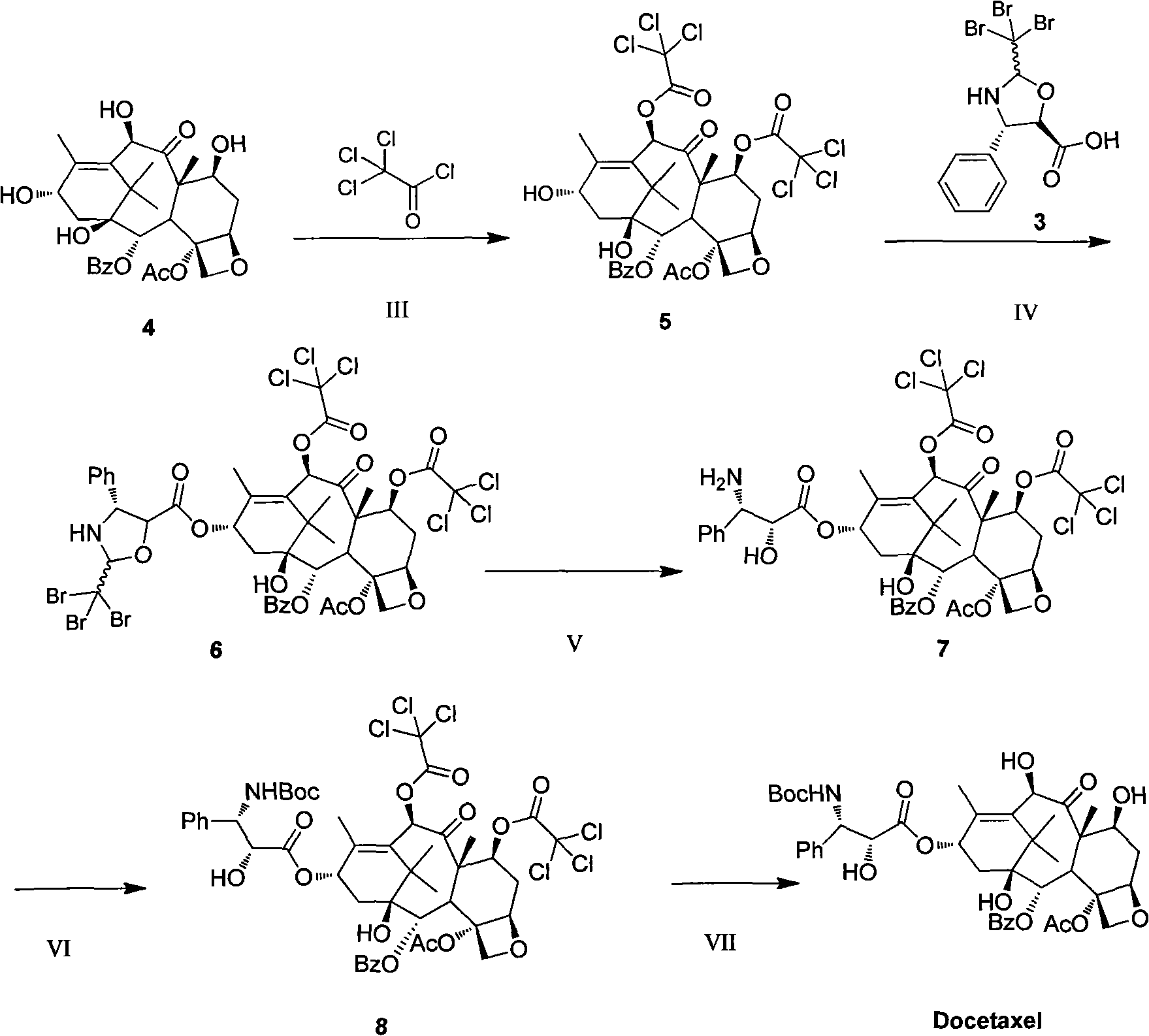
[0034]
[0035] Dissolve 10-DAB (544.6 mg, 1 mmol) in 5 mL of anhydrous pyridine, under nitrogen protection, under ice-salt bath conditions, slowly add trichloroacetyl chloride (600.0 mg, 3.3 mmol) dropwise, the temperature is controlled at minus 5 degrees, three After the dropwise addition of chloroacetyl chloride was completed, it was naturally raised to room temperature and detected by TLC. After 1.5 hours, the raw material disappeared. Stir and drop in deionized water under ice bath conditions, extract with ethyl acetate, dry with anhydrous sodium sulfate, filter, and reduce pressure The solvent was distilled off, and compound 5 was obtained by column chromatography: 1.08 g, with a yield of 96%.
[0036] 1 H-NMR (400MHz, CDCl 3 ): δ7.67(t, 2H), 7.60(t, 1H), 7.47(t, 2H), 6.27(s, 1H), 5.66-5.60(m, 2H), 4.99(d, 1H), 4.92( d, 1H), 4.60(d, 1H), 4.32(d, 1H), 4.15(d, 1H), 3.98(d, 1H), 2.64-2.62(m, 1H), 2.30(s, 3H), 2.16 (s, 3H), 2.15-1.86(m, 2H), 1.84(s, 3H), 1.14(s, 3H), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com