Preparation method of cefalexin capsule
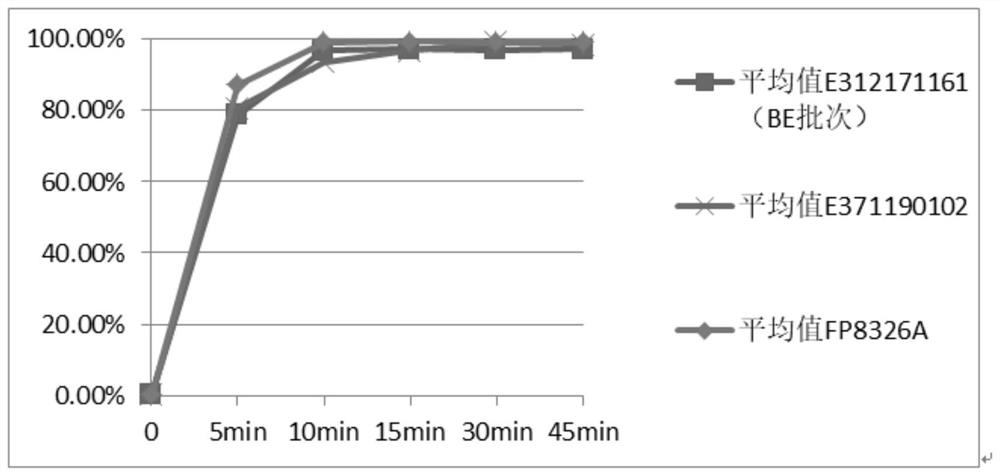
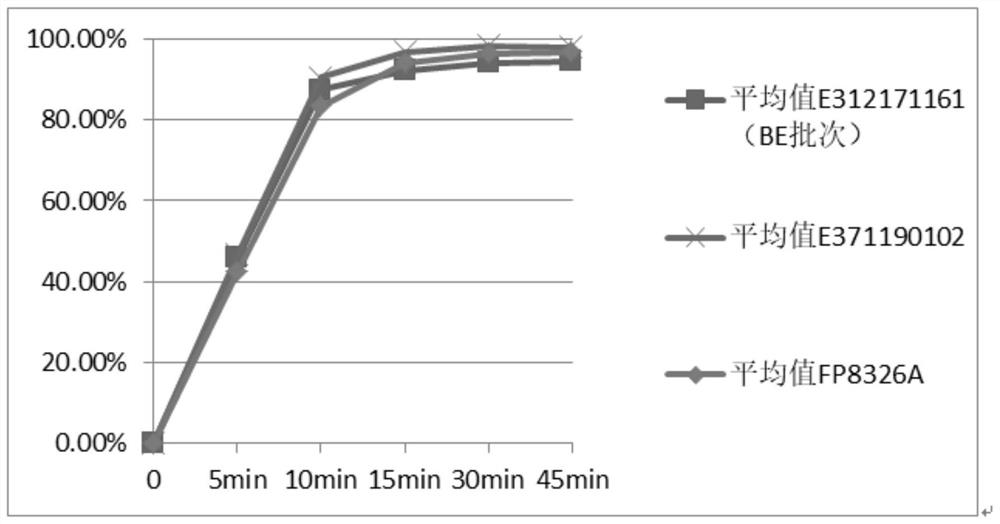
A technology for cephalexin capsules and cephalexin, which is applied in the field of chemical medicine and can solve the problems of low similarity of the dissolution curve of the product and the reference preparation, the detection method not conforming to the quality research, and the inequivalence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
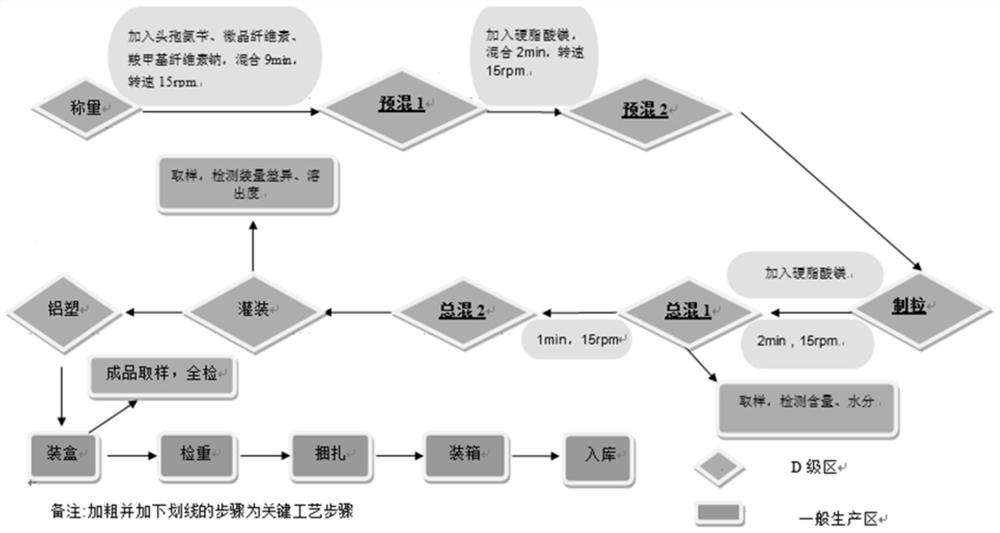
[0027] Example 1: 0.25g specification production verification batch / clinical experiment batch, batch size 600,000 capsules / batch, batch number E312171161
[0028] A. Weigh the cephalexin raw material (150kg in dry form), microcrystalline cellulose-carboxymethyl cellulose sodium compound and magnesium stearate, first mix the cephalexin raw material, microcrystalline cellulose-carboxymethyl Add the cellulose sodium compound into the hopper, set the mixing speed at 15rpm / min, and the mixing time is 9min. After the mixing is completed, pause, add the internally added magnesium stearate and continue mixing for 5min at the mixing speed of 15rpm / min.
[0029] B. After the mixing is completed, the materials are added to a dry granulator for dry granulation, and the calculated yield is 95.35% after the granulation is completed.
[0030] C. Weigh the added magnesium stearate and add it into the hopper, set the mixing speed to 15rpm / min, and the mixing time to 3min, and store it in the i...
Embodiment 2
[0033] Example 2: 0.125g specification production verification batch, batch size 1.2 million grains / batch, batch number E371190102
[0034] A. Weigh the cephalexin raw material (150kg in dry form), microcrystalline cellulose-carboxymethyl cellulose sodium compound and magnesium stearate, first mix the cephalexin raw material, microcrystalline cellulose and carboxymethyl cellulose Sodium cellulose was added into the hopper, the mixing speed was set to 15rpm / min, and the mixing time was 9min. After the mixing was completed, it was paused, and after adding the internally added magnesium stearate, the mixing was continued for 5min, and the mixing speed was 15rpm / min.
[0035] B. After the mixing is completed, the materials are added to the dry granulator for dry granulation, and the calculated yield after the granulation is 99.1%.
[0036] C. Weigh the added magnesium stearate and add it to the hopper, set the mixing speed to 15rpm / min, and the mixing time to 3min, and store it in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com