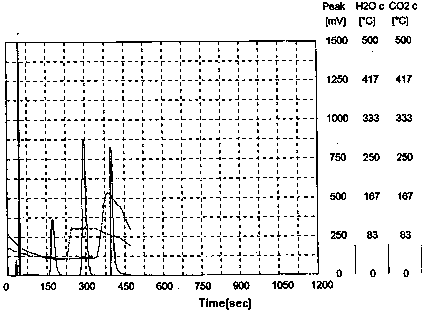
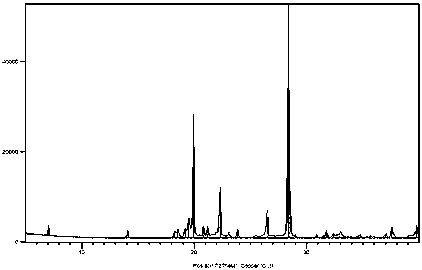
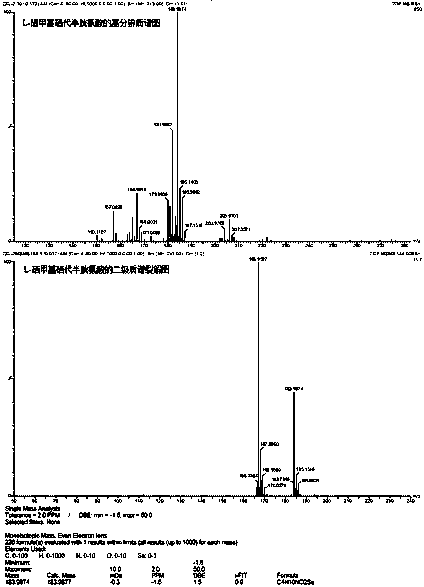
Green and efficient method for preparing L-selenium methylselenocysteine
A technology of selenocysteine and selenomethyl, applied in the field of green and efficient preparation of L-selenomethyl selenocysteine, which can solve the problems of harsh reaction conditions, environmental pollution, and high equipment requirements, and meet the requirements of the reaction conditions Moderate, avoid environmental pollution, overcome the effect of instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A green and efficient method for preparing L-selenomethylselenocysteine, comprising the following steps:
[0058] (a) Add 50 g of L-serine methyl ester hydrochloride to 300 mL of tetrahydrofuran under stirring condition, and cool to 0~5°C;
[0059] (b) Add 70.8 g of triethylamine, stir well, then add 26.5 g of acetyl chloride dropwise (about 1 h to complete the dropwise addition), and continue to stir for 3 h after the dropwise addition; then slowly add 34.4 g of thionyl chloride dropwise ( 0.5 h), continue to stir for 1 h after the dropwise addition, raise the temperature to 45°C, stir for 3 h, then cool down to 0°C;
[0060] (c) Add 600 mL of saturated sodium bicarbonate solution, stir for 0.5 h, then add dropwise 300 mL of sodium methylselenide in tetrahydrofuran (containing 37.3 g of sodium methylselenide), dropwise for about 2 hours, continue stirring for 3 hours , warmed up to room temperature, and stirred for 12 h to obtain a reaction mixture;
[0061] (d) Afte...
Embodiment 2
[0082] A green and efficient method for preparing L-selenomethylselenocysteine, comprising the following steps:
[0083] (a) Add 50 g of L-serine methyl ester hydrochloride to 300 mL of tetrahydrofuran under stirring condition, and cool to 0~5°C;
[0084] (b) Add 70.8 g of triethylamine, stir evenly, then add 26.5 g of acetyl chloride dropwise (about 1 h to complete the dropwise addition), and continue to stir for 4 h after the dropwise addition; then slowly add 36.1 g of thionyl chloride dropwise ( 0.5 h), continue to stir for 1 h after the dropwise addition, raise the temperature to 45°C, stir for 3 h, then cool down to 0°C;
[0085] (c) Add 600 mL of saturated sodium bicarbonate solution, stir for 0.5 h, then add dropwise 300 mL of sodium methylselenide in tetrahydrofuran (containing 37.3 g of sodium methylselenide), dropwise for about 2 hours, continue stirring for 3 hours , warmed up to room temperature, and stirred for 12 h to obtain a reaction mixture;
[0086] (d) Af...
Embodiment 3
[0097] A green and efficient method for preparing L-selenomethylselenocysteine, comprising the following steps:
[0098] (a) Add 50 g of L-serine methyl ester hydrochloride to 300 mL of tetrahydrofuran under stirring condition, and cool to 0~5°C;
[0099] (b) Add 70.8 g of triethylamine, stir evenly, then add 27.8 g of acetyl chloride dropwise (about 1 h to complete the dropwise addition), and continue to stir for 4 h after the dropwise addition; then slowly add 37.8 g of thionyl chloride dropwise ( 0.5 h), continue to stir for 1 h after the dropwise addition, raise the temperature to 45°C, stir for 3 h, then cool down to 0°C;
[0100] (c) Add 600 mL of saturated sodium bicarbonate solution, stir for 0.5 h, then add dropwise 300 mL of sodium methylselenide in tetrahydrofuran (containing 37.3 g of sodium methylselenide), dropwise for about 2 hours, continue stirring for 3 hours , warmed up to room temperature, and stirred for 12 h to obtain a reaction mixture;
[0101] (d) Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com