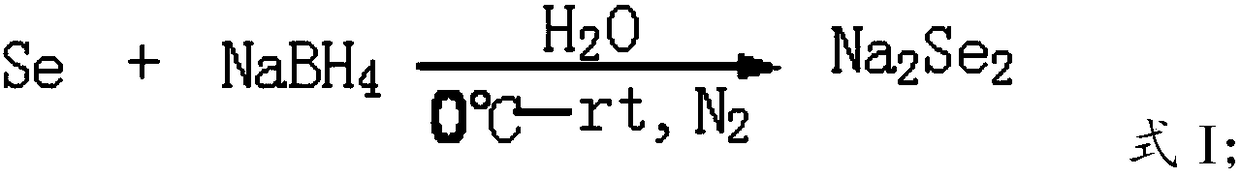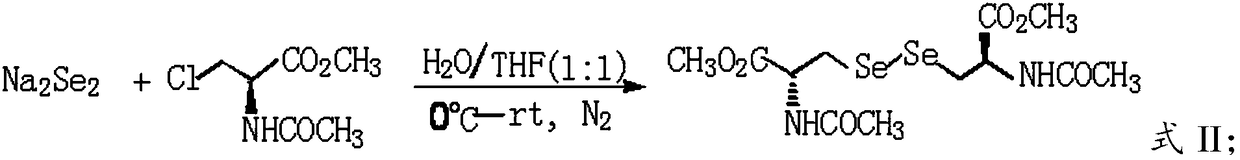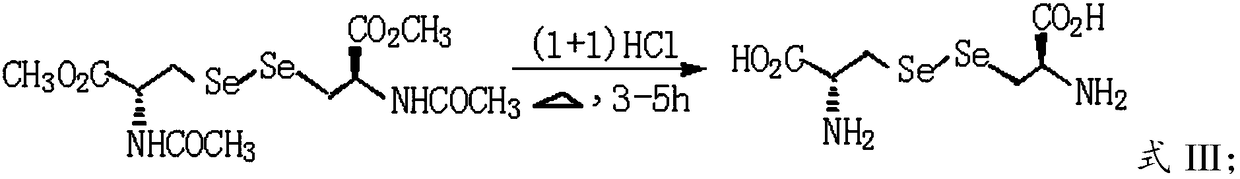Preparation method of L-selenocystine
A technology for selenocystine and dimethylcystine, which is applied in the field of preparation of L-selenocystine, can solve the problems of difficult separation and purification of substituted products, complicated processes, and tediousness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a kind of preparation method of L-selenocystine, comprises the following steps:
[0028] A) carrying out nucleophilic substitution reaction between sodium diselenide and N-acetyl-3-chloro-L-serine methyl ester to obtain N,N'-diacetyl-L-selenocystine dimethyl ester;
[0029] B) Hydrolyzing N,N'-diacetyl-L-selenocystine dimethyl ester in hydrochloric acid solution to obtain L-selenocystine.
[0030] The present invention preferably prepares sodium diselenide according to the following steps, and then performs nucleophilic substitution reaction between sodium diselenide and N-acetyl-3-chloro-L-serine methyl ester to obtain N,N'-diacetyl- L-Selenocystine Dimethyl Ester;
[0031] Under the conditions of ice bath and nitrogen protection, elemental selenium and sodium borohydride are reacted in a solvent to obtain sodium diselenide. The present invention preferably now mixes a part of elemental selenium with a solvent, then adds sodium borohydride unde...
Embodiment 1
[0052] Preparation of L-selenocystine using N-acetyl-3-chloro-L-serine methyl ester:
[0053] Add 30g of selenium powder and 500mL of water into a 2L flask equipped with an electric stirrer, a reflux condenser, and an air guide tube, stir to make it evenly mixed, and then pour N 2 Under protection and ice bath, slowly add 30g of sodium borohydride for about 0.5h, raise to room temperature for 20min, then add 30g of selenium powder, react at room temperature for 20min, rise to 70°C for 20min to obtain a reddish-brown solution ;
[0054] Cool the above solution to about 0°C, add 500mL tetrahydrofuran, stir evenly, add 120g N-acetyl-3-chloro-L-serine methyl ester, continue the reaction for 4h, then raise the temperature to 37°C for 12h until N-acetyl-3- Chloro-L-serine methyl ester was completely reacted (TLC detection), adjusted to pH<5.0 with 6M HCl, extracted three times with ethyl acetate, combined organic phases, washed with water, dried over anhydrous sodium sulfate, filte...
Embodiment 2
[0064] Preparation of L-selenocystine using N-acetyl-3-chloro-L-serine methyl ester:
[0065] Add 30g of selenium powder and 500mL of water into a 2L flask equipped with an electric stirrer, a reflux condenser, and an air guide tube, stir to make it evenly mixed, and then pour N 2 Under protection and ice bath, slowly add 30g of sodium borohydride for about 0.5h, raise to room temperature for 20min, then add 30g of selenium powder, react at room temperature for 20min, rise to 70°C for 20min to obtain a reddish-brown solution ;
[0066] Cool the above solution to about 0°C, add 500mL tetrahydrofuran, stir evenly, add 100g N-acetyl-3-chloro-L-serine methyl ester, continue the reaction for 4h, then raise the temperature to 37°C for 12h until N-acetyl-3- The reaction of chloro-L-serine methyl ester was complete (TLC detection), adjusted the system pH<5.0 with 6M HCl, extracted three times with ethyl acetate, combined the organic phases, washed with water, dried over anhydrous sod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com