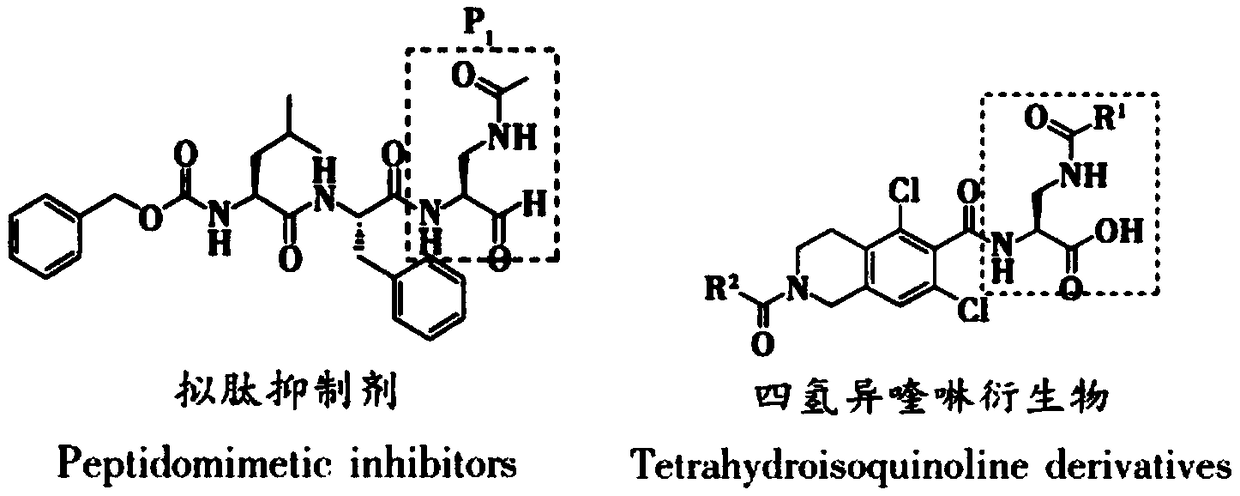
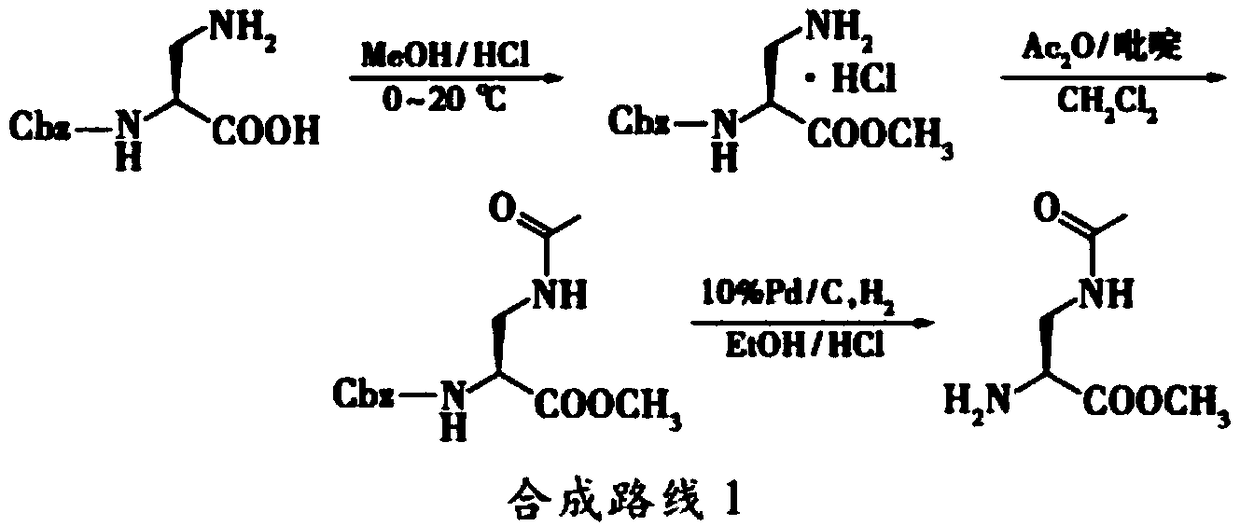
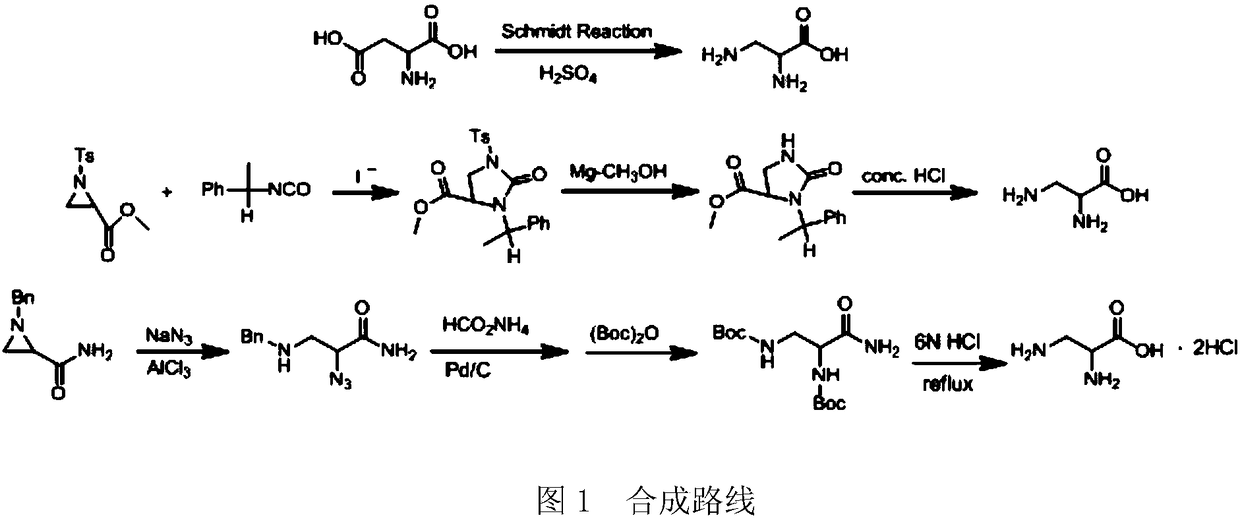
Preparation method of 2,3-diamido methyl propionate
A technology of methyl diaminopropionate and methyl serine, which is applied in the field of compound preparation, can solve problems such as mild reaction conditions, cumbersome synthetic routes, and difficulty in obtaining starting materials, and achieve the effect of mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In this example, serine is used as a raw material, and the active ester generated by thionyl chloride and methanol is used in methanol to prepare serine methyl ester, which is then reacted with triphenylchloromethane to generate intermediate product 1, and the Trt group is introduced. Intermediate product 1 is Anhydrous tetrahydrofuran as a solvent, under the catalysis of triphenylphosphine and diisopropyl azodicarboxylate DIAD (molecular formula: (CH3)2CHOOCN=NCOOCH(CH3)2), reacts with phthalimide to form The Pht group was introduced into the intermediate product 2, the Pht group was removed from the intermediate product 2 under the action of hydrazine hydrate to generate the intermediate product 3, and the Trt group was finally removed from the intermediate product 3 in hydrochloric acid ethanol to obtain the final product 4.
[0038] The specific process is as follows:
[0039] (1) Synthesis of serine methyl ester:
[0040] Add 30ml of methanol to a 100ml flask, sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com