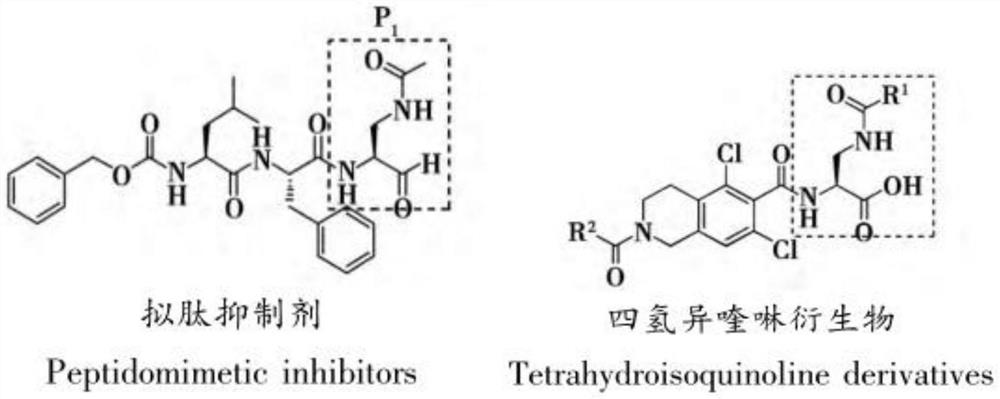
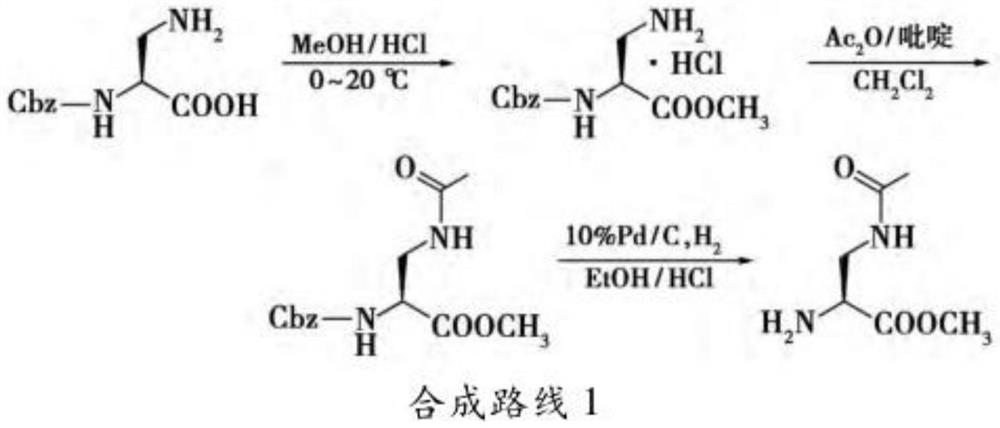
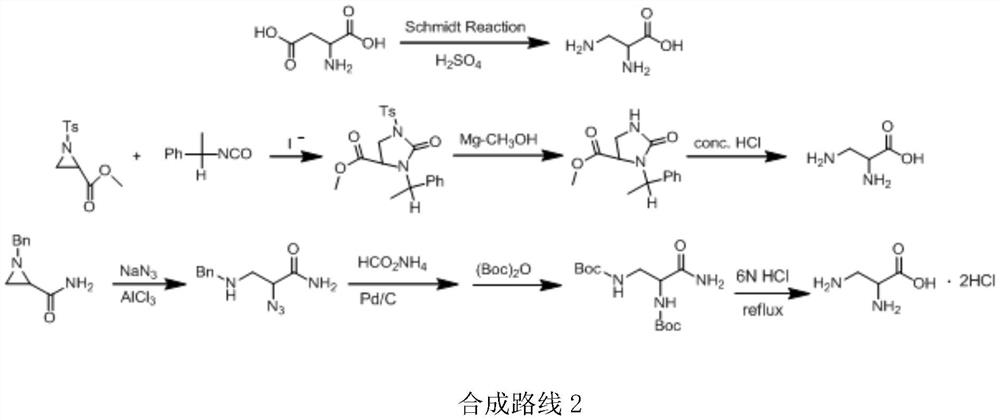
A kind of preparation method of 2,3-diaminopropionic acid methyl ester
A technology of methyl diaminopropionate and methyl serine, which is applied in the field of compound preparation, can solve problems such as cumbersome synthetic routes, mild reaction conditions, and difficulty in obtaining starting materials, and achieve high yield and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In this example, serine is used as a raw material, and the active ester generated by thionyl chloride and methanol is used in methanol to prepare serine methyl ester, which is then reacted with triphenylchloromethane to generate intermediate product 1, and the Trt group is introduced. Intermediate product 1 is Anhydrous tetrahydrofuran is solvent, in triphenylphosphine and diisopropyl azodicarboxylate DIAD (molecular formula is: (CH 3 ) 2 CHOOCN=NCOOCH(CH 3 ) 2 ) under the catalysis, react with phthalimide to generate intermediate product 2, introduce Pht group, intermediate product 2 removes Pht group to generate intermediate product 3 under the action of hydrazine hydrate, and finally intermediate product 3 is in hydrochloric acid ethanol Removal of the Trt group afforded the final product 4.
[0038] The specific process is as follows:
[0039] (1) Synthesis of serine methyl ester:
[0040] Add 30ml of methanol to a 100ml flask, stir in an ice-water bath, slowly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com