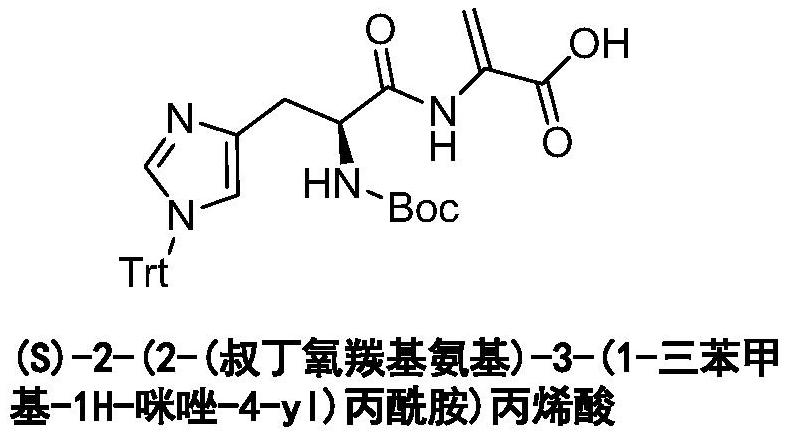
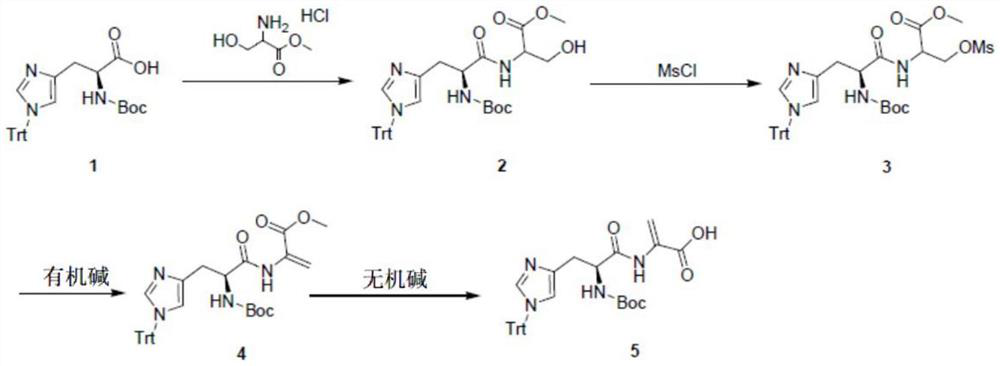
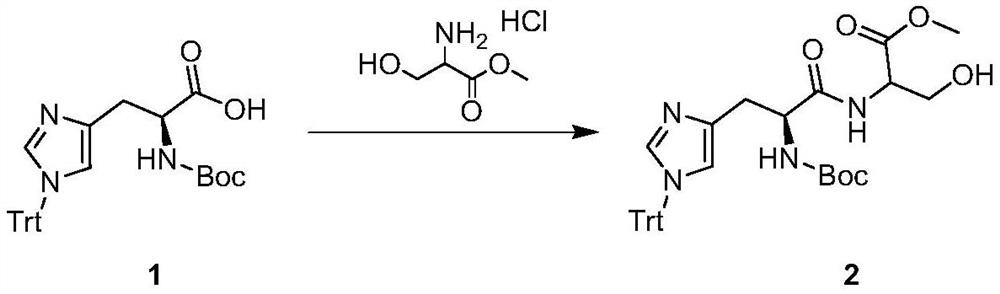
A kind of synthetic method of acrylic acid derivative
A synthesis method and a derivative technology, applied in the direction of organic chemistry, etc., can solve the problem of no literature report in the synthesis, and achieve the effect of simple reaction operation and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Methyl 2-((S)-2-(tert-butoxycarbonylamino)-3-(1-trityl-1H-imidazole-4-yl)propionamide)-3-hydroxypropionate preparation of
[0023]
[0024] Dissolve (S)-2-(tert-butoxycarbonylamino)-3-(1-trityl-1H-imidazole-4-yl)propionic acid 2200g (4.42mol, 1.0eq) in dimethylformamide In 15L, add pyridine 1042g (13.17mol, 3.0eq), add serine methyl ester hydrochloride 753g (4.84mol, 1.1eq); add EDCI 1011g (5.30mol, 1.2eq) in batches, control the internal temperature to less than 15°C, After addition, react at 20-30°C for 3h. The reaction solution was poured into 45 L of ice water, and a large amount of solids precipitated out. Filter, dissolve the filter cake with 15L of EA / THF=5 / 1, wash once with 5L of 1N HCl, wash once with 5L of saturated saline, and dry sodium sulfate. Filter, concentrate the organic phase under reduced pressure until a large amount of solids precipitate, add 5L of petroleum ether to make a slurry, filter, and dry to obtain 2-((S)-2-(tert-butoxycar...
Embodiment 2
[0025] Example 2: 2-((S)-2-(tert-butoxycarbonylamino)-3-(1-trityl-1H-imidazole-4-yl)propionamide)-3-methanesulfonyloxy Preparation of methyl propionate:
[0026]
Embodiment 3
[0028] Example 3: Preparation of (S)-2-(2-(tert-butoxycarbonylamino)-3-(1-trityl-1H-imidazole-4-yl)propionamide)methyl acrylate:
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com