Solid pharmaceutical formulations of ramipril and amlodipine besylate, and their preparation
A kind of technology of amlodipine besylate and amlodipine besylate, applied in the directions of drug combination, drug delivery, medical preparations containing active ingredients, etc., can solve problems such as low content of amlodipine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation of stable pharmaceutical formulations of ramipril is complicated because of its susceptibility to certain types of pH-dependent degradation.
[0060] The formation of ramipril DKP is mainly dependent on the pH of the formulation, especially at very low pH values. It is reported in the literature that increasing the pH of the microenvironment of ramipril minimizes the formation of DKP impurities.
[0061] However, regarding amlodipine, the photodegradation product impurity-D of amlodipine was more formed in alkaline environment and less in weak acid environment. Istin tablets have a pH of 7.2 and Cardace has a pH of ~4.2-4.3. (In contrast, pH 4.7-5.0 in ramipril + amlodipine tablets).
[0062] Extragranular amlodipine besylate (special dry blended), which is naturally alkaline, was included in the FDC product to impart a pH of 4.7-5.0 to the FDC tablet. Therefore, the stability of ramipril in the combination formulation is improved.
[0063] Likewise,...
Embodiment 1
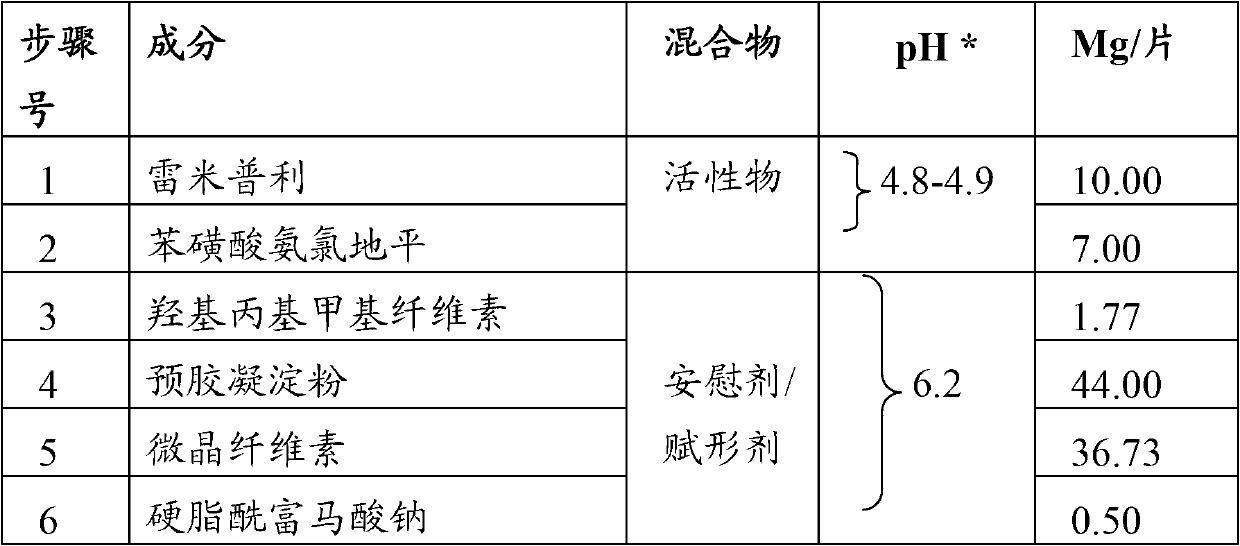
[0126] Example 1. Composition using a placebo with a pH of 6.2
[0127]
[0128] *Amount of material taken and dispersed in 50ml of purified water
[0129] step:
[0130] 1. Ramipril added as hydroxypropyl methylcellulose granules
[0131] 2. Geometrically mix ramipril granules with pregelatinized starch.
[0132] 3. Mix amlodipine besylate and microcrystalline cellulose geometrically and mix them with the material from step 2 and mix in a mixer for 20 minutes to achieve homogeneity.
[0133] 4. Lubricate the mixture with sodium stearyl fumarate.
[0134] 5. Compress the mixture into tablets using suitable punches on the tablet press.
[0135] 6. Tablets were packaged in opaque white double-layer packaging and subjected to a 6-month accelerated stability study, analyzing the percentage of potential degradants at the initial and 6-month intervals
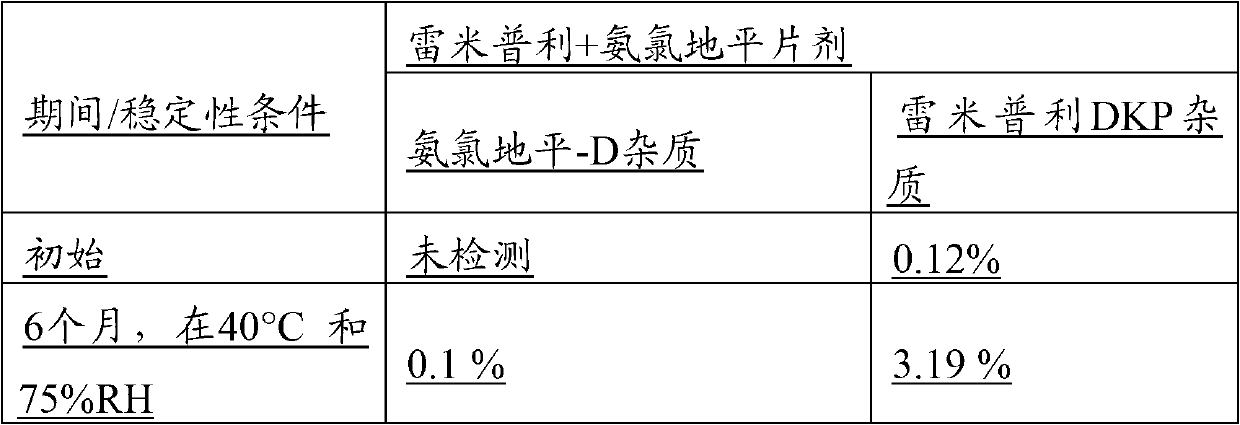
[0136] Table 1
[0137]
[0138] Formulations In a further trial, placebo pH studies of the stability of the two active...
Embodiment 2
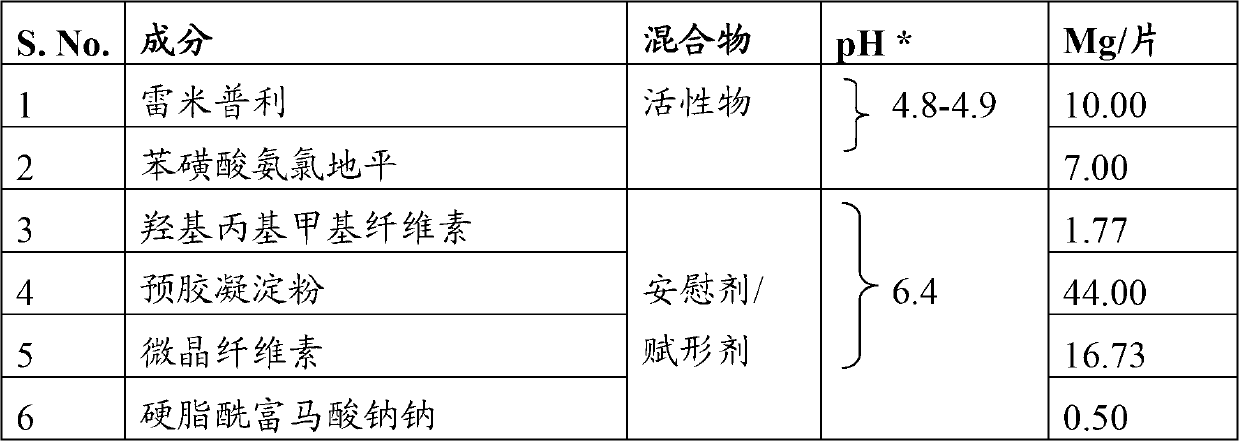
[0139] Example 2: Composition using placebo at pH 6.40
[0140]
[0141] *Amount of material taken and dispersed in 50ml of purified water
[0142] step:
[0143] 1. Ramipril added as hydroxypropyl methylcellulose granules
[0144] 2. Geometrically mix ramipril granules and microcrystalline cellulose
[0145] 3. Mix amlodipine besylate and pregelatinized starch geometrically and mix them with the material from step 2 and mix in a mixer for 25 minutes to achieve homogeneity.
[0146] 4. Lubricate the mixture with sodium stearyl fumarate.
[0147] 5. Compress the mixture into tablets using suitable punches on the tablet press.
[0148] 6. Tablets were packaged in opaque white double-layer packaging and subjected to a 6-month accelerated stability study, analyzing the percentage of potential degradants at the initial and 6-month intervals
[0149] Table 2
[0150]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com