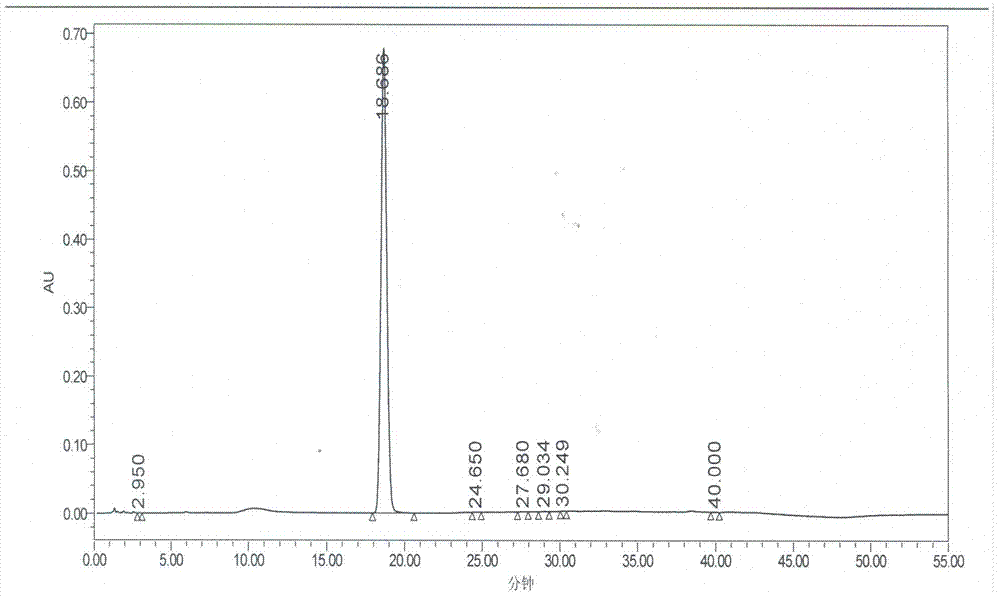
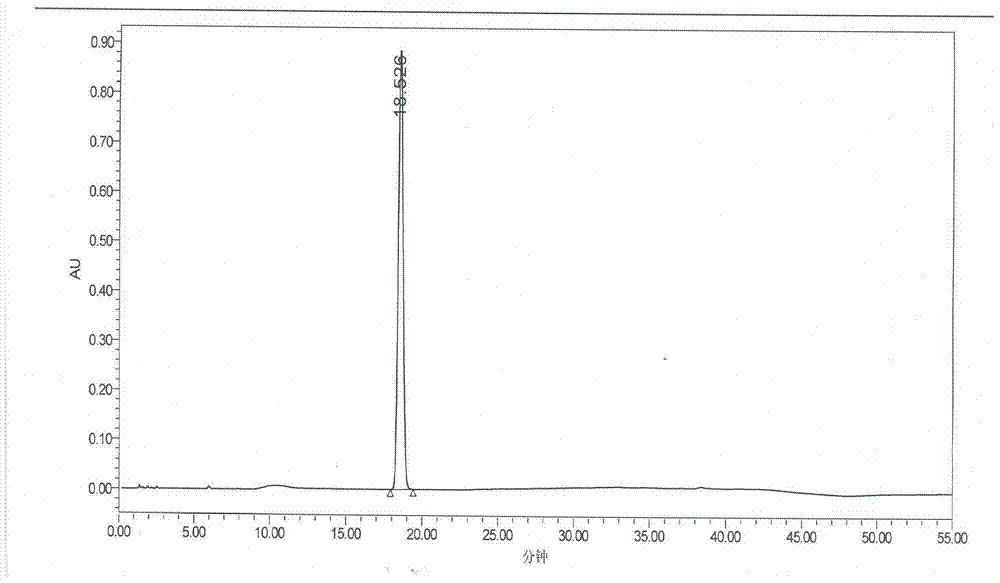
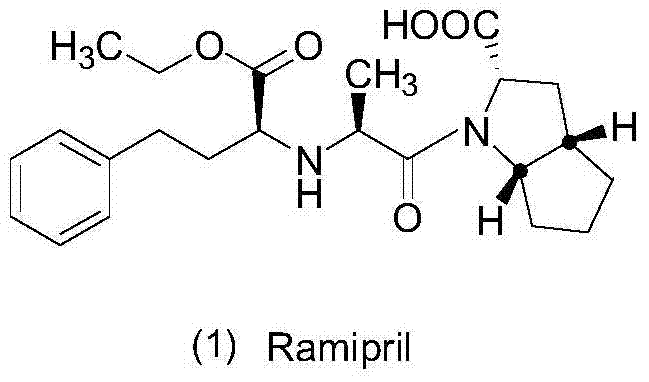
Preparation method of ramipril
A compound and organic base technology, applied in the field of preparation of ramipril as an ACE inhibitor drug, can solve the problems of difficult separation of isomers, ineffective effects, and increased industrialization costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 preparation of benzyl ramipril
[0032] Add dichloromethane (250L), N-[1-(s)-ethoxycarbonyl-3-phenylpropyl]-L-alanine (17.5kg), (s,s,s) to the reaction kettle in sequence -2-Azabicyclo[3,3,0]octane-3-carboxylic acid benzyl ester hydrochloride (17.7kg), DCC (15.5kg), HOBt (8.5kg), stirring (210rpm), temperature control 10 ℃; slowly add triethylamine (13L) to the above kettle, and finish adding in about 1 hour, and control the temperature at about 10 ℃; after adding triethylamine, keep warm at about 10 ℃ and stir for 6 hours; ; Slowly add 50L0.5mol / L hydrochloric acid to the filtrate, stir rapidly (300rpm), stir and react for 1h; centrifuge and filter, separate liquid, collect organic phase; use saturated sodium bicarbonate solution to wash 3 times (200L / time); separate liquid, Collect the organic phase and wash it once with deionized water (200L / time); separate the liquid, collect the organic phase, and concentrate under reduced pressure (30°C-40°C, vacuum ...
Embodiment 2
[0033] Embodiment 2 preparation of benzyl ramipril
[0034]Add dichloromethane (250L), N-[1-(s)-ethoxycarbonyl-3-phenylpropyl]-L-alanine (16.5kg), (s,s,s) to the reaction kettle in sequence -2-Azabicyclo[3,3,0]octane-3-carboxylate benzyl sulfate (19.5kg), DCC (16.3kg), HOBt (7.3kg), stirring (240rpm), temperature control 15℃ About; slowly add triethylamine (15L) to the above kettle, and finish adding in about 1.5h, and control the temperature at about 16°C; after adding triethylamine, keep warm at about 10°C and stir for 6h; ; Slowly add 45L0.5mol / L hydrochloric acid to the filtrate, stir rapidly (300rpm), stir and react for 2h; centrifuge and filter, separate liquid, collect organic phase; use saturated sodium bicarbonate solution to wash 3 times (250L / time); separate liquid, Collect the organic phase and wash it once with deionized water (250L / time); separate the liquid, collect the organic phase, and concentrate under reduced pressure (30°C-40°C, vacuum degree-0.08Mpa-0.1M...
Embodiment 3
[0035] Embodiment 3 Ramipril crude product preparation
[0036] Transfer the oil obtained in Example 1 to a hydrogenation reactor, add 95% ethanol (200L), 10% palladium carbon (1.8kg), and seal it; nitrogen replaces the air in the reactor; after the replacement, feed hydrogen (pressure 0.4MPa- 0.45MPa), the temperature is controlled at about 15°C, the stirring is started, and the heat preservation and pressure reaction is carried out; the hydrogen pressure indication number has not decreased, and the hydrogenation is continued for about 3.5h; the reaction is completed, exhausted, and replaced by nitrogen; The filtrate was collected; the filtrate was concentrated under reduced pressure (30°C-40°C, vacuum degree -0.08Mpa-0.1Mpa); after concentration, an oily substance (20.7kg, yield 82.6%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com