Camptothecin and artesunate conjugate, preparation method and application thereof
A technology of artesunate and conjugates, which is applied in the field of preparation of antitumor drugs, can solve the problems of poor solubility and high toxicity, and achieve the effect of reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of camptothecin and artesunate conjugates (1)
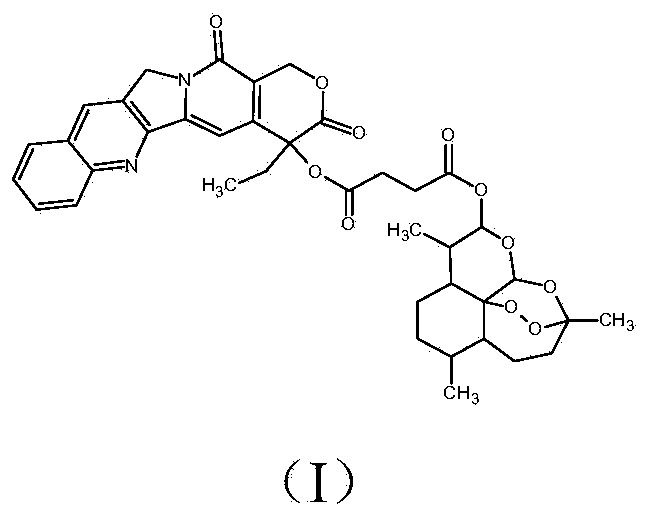
[0028] Add artesunate (15mmol) and dichloromethane (10mL) into a 25mL round bottom flask, stir to dissolve at 25°C, then add EDCI (15mmol), stir at 25°C for 0.5h, then add DMAP (50mg), stir at 25°C After 0.5h, camptothecin (5mmol) was finally added, stirred and reacted at 25°C for 3h to obtain a light yellow suspension. Sodium aqueous solution and saturated sodium bicarbonate aqueous solution were washed, then dried with anhydrous magnesium sulfate, suction filtered, the filtrate was concentrated to dryness under reduced pressure, and the obtained concentrate was subjected to silica gel column chromatography, and the eluent was dichloromethane with a volume ratio of 100:1 Mix the liquid with methanol, collect the eluate containing the target component, concentrate and dry to obtain the conjugate of camptothecin and artesunate represented by formula (I), with a yield of 80%.
[0029] MP:194.8-195.3℃ ...
Embodiment 2
[0030] Example 2: Preparation of camptothecin and artesunate conjugates (2)
[0031] Add artesunate (10mmol) and chloroform (20mL) into a 25mL round bottom flask, stir to dissolve at 30°C, then add EDCI (10mmol), stir at 30°C for 0.5h, then add DMAP (50mg), stir at 25°C for 0.5h , finally add camptothecin (3mmol), stir and react at 30°C for 3h to obtain a light yellow suspension, after the reaction, add dichloromethane (40mL) to the reaction solution, filter with suction, and the filtrate is successively washed with saturated aqueous sodium chloride solution and saturated aqueous sodium bicarbonate solution, then dried with anhydrous magnesium sulfate, suction filtered, and the filtrate was concentrated to dryness under reduced pressure, and the obtained concentrate was subjected to silica gel column chromatography, and the eluent was dichloromethane and methanol at a volume ratio of 100:1 For the mixed solution, the eluate containing the target component was collected, concen...
Embodiment 3
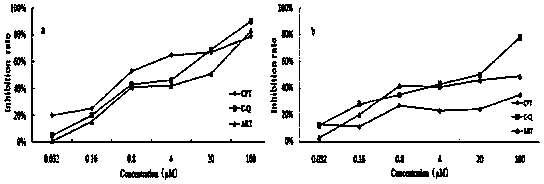
[0032] Embodiment 3: The compound shown in formula (I) has inhibitory activity on tumor cell proliferation
[0033] 1) Test material
[0034] Liver cancer cells (SMMC-7721) and human breast cancer cells (MCF-7) were purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences.
[0035] Standard products of camptothecin and artesunate were purchased from Bailingwei Biotechnology Co., Ltd.
[0036] The cell maintenance medium is 2% serum concentration medium, RPMI1640 medium is used for SMMC-7721, and DMEM medium is used for MCF-7.
[0037] The camptothecin and artesunate conjugate prepared in Example 1, camptothecin, artesunate, dimethyl sulfoxide (DMSO) were diluted with cell maintenance solution respectively, and 6 concentration gradients were made for each sample : 0.032, 0.16, 0.8, 4, 20, 100 μmol / L, 4 replicate wells for each concentration gradient.
[0038] 2) Detection method
[0039] Human liver cancer cells (SMMC-7721) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com