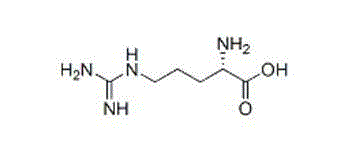
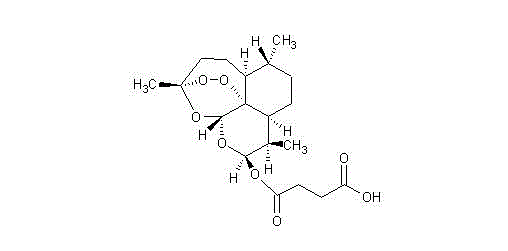
Artesunate and L-arginine composition for injection and preparation method thereof
A technology of artesunate and composition, which is applied in the field of drug preparation, can solve the problems of unstable sodium bicarbonate solution, low solubility of artesunate in water, safety risks, etc., and achieve easy and convenient preparation, increase the scope of use, The effect of stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
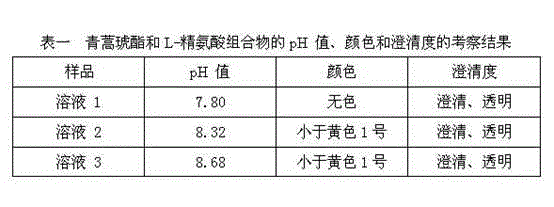
[0027] Prescription: Artesunate 1000 parts, L-arginine 450 parts;
[0028] The preparation steps are as follows:
[0029] (1) After the raw material sterile artesunate and the auxiliary material L-arginine are removed from the outer packaging, they are dust-cleaned, wiped and sterilized, and put into a sterile room for standby;
[0030] (2) In the 100-level clean area, artesunate and L-arginine were crushed separately and passed through an 80-mesh sieve;
[0031] (3) Weigh the artesunate and L-arginine obtained in step (2) in a 100-level clean area, mix them evenly according to the prescription ratio, and pack them into control bottles after the measured content is qualified, cork and cap , Light inspection, inspection qualified, labeling, packaging.
Embodiment 2
[0033] Prescription: Artesunate 1000 parts, L-arginine 900 parts;
[0034] Preparation steps:
[0035] (1) After the raw material sterile artesunate and auxiliary material L-arginine are removed from the outer packaging, dust is removed, cleaned, wiped and sterilized, and put into a sterile room for standby;
[0036] (2) In the 100-level clean area, artesunate and L-arginine were crushed separately and passed through an 80-mesh sieve;
[0037] (3) Weigh the artesunate and L-arginine obtained in step (2) in a 100-level clean area, mix them evenly according to the prescription ratio, and pack them into control bottles after the measured content is qualified, cork and cap , Light inspection, inspection qualified, labeling, packaging.
Embodiment 3
[0039] Prescription: Artesunate 1000 parts, L-arginine 675 parts;
[0040] The preparation steps are as follows:
[0041] (1) Weigh artesunate and L-arginine according to the prescription ratio and add them to the water for injection, then add an appropriate amount of mannitol, stir to dissolve, and sterile filter into the sterile room for standby;
[0042] (2) After the semi-finished product passes the inspection, it is divided into control bottles and waits for freeze-drying;
[0043] (3) Freeze drying process:
[0044] 1) Pre-freezing: first pre-lower the temperature of the condenser to below -50°C, and then quickly drop the temperature of the plate layer to below -35°C after the product enters the box, and keep it in a low temperature state for 2 hours;
[0045] 2) Primary drying: Turn on the vacuum pump, keep the vacuum at 10-15Pa, slowly raise the temperature to 4-6°C, keep it warm for 5-8 hours, and perform primary drying;
[0046] 3) Secondary drying: raise the temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com