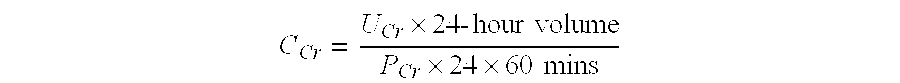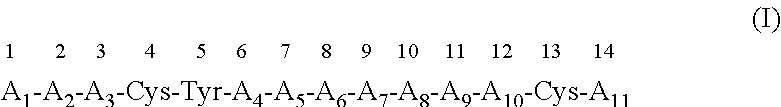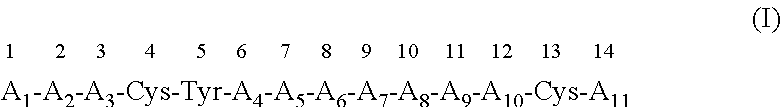Patents
Literature
170 results about "CD40" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cluster of differentiation 40, CD40 is a costimulatory protein found on antigen presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.
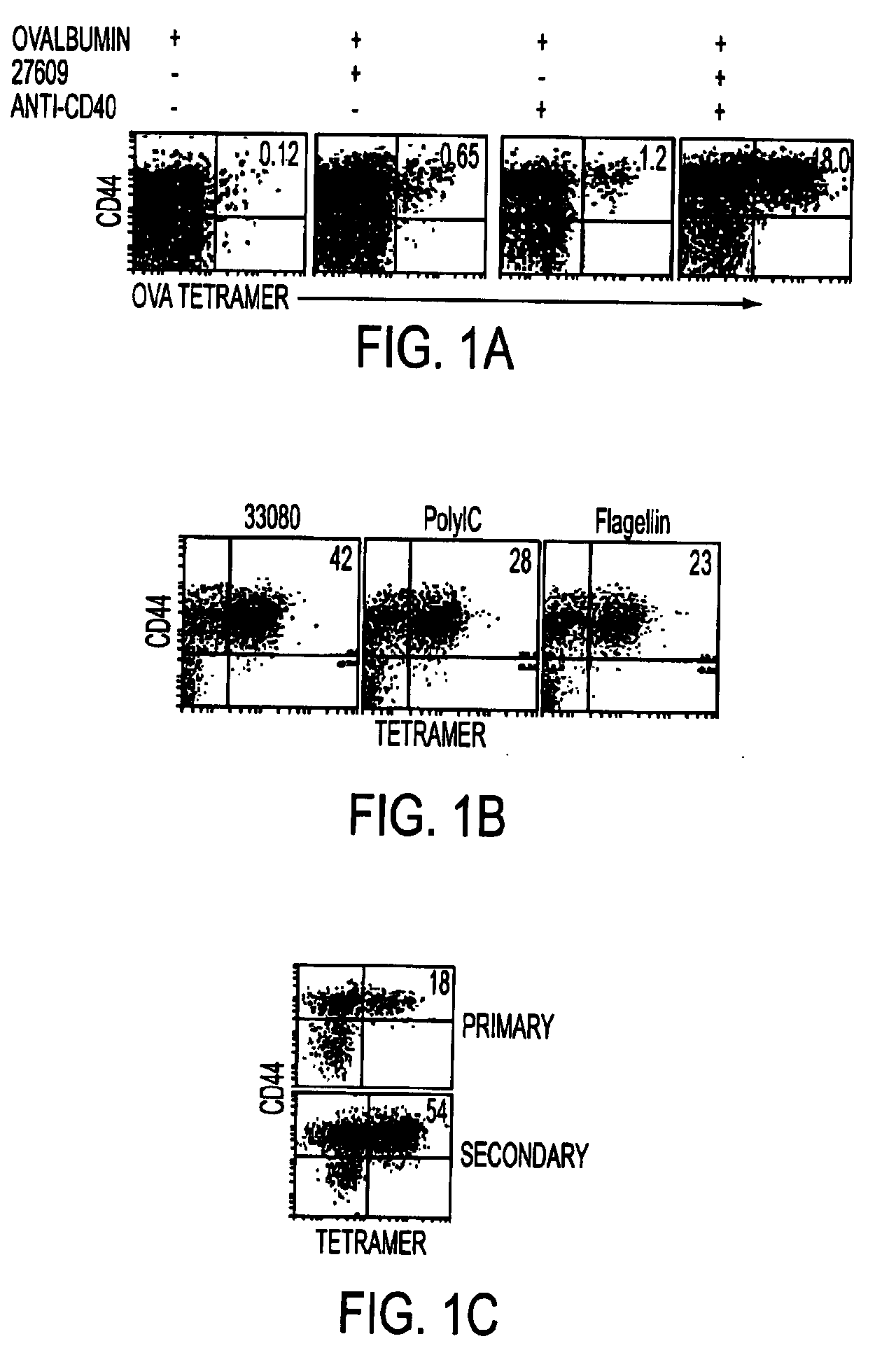
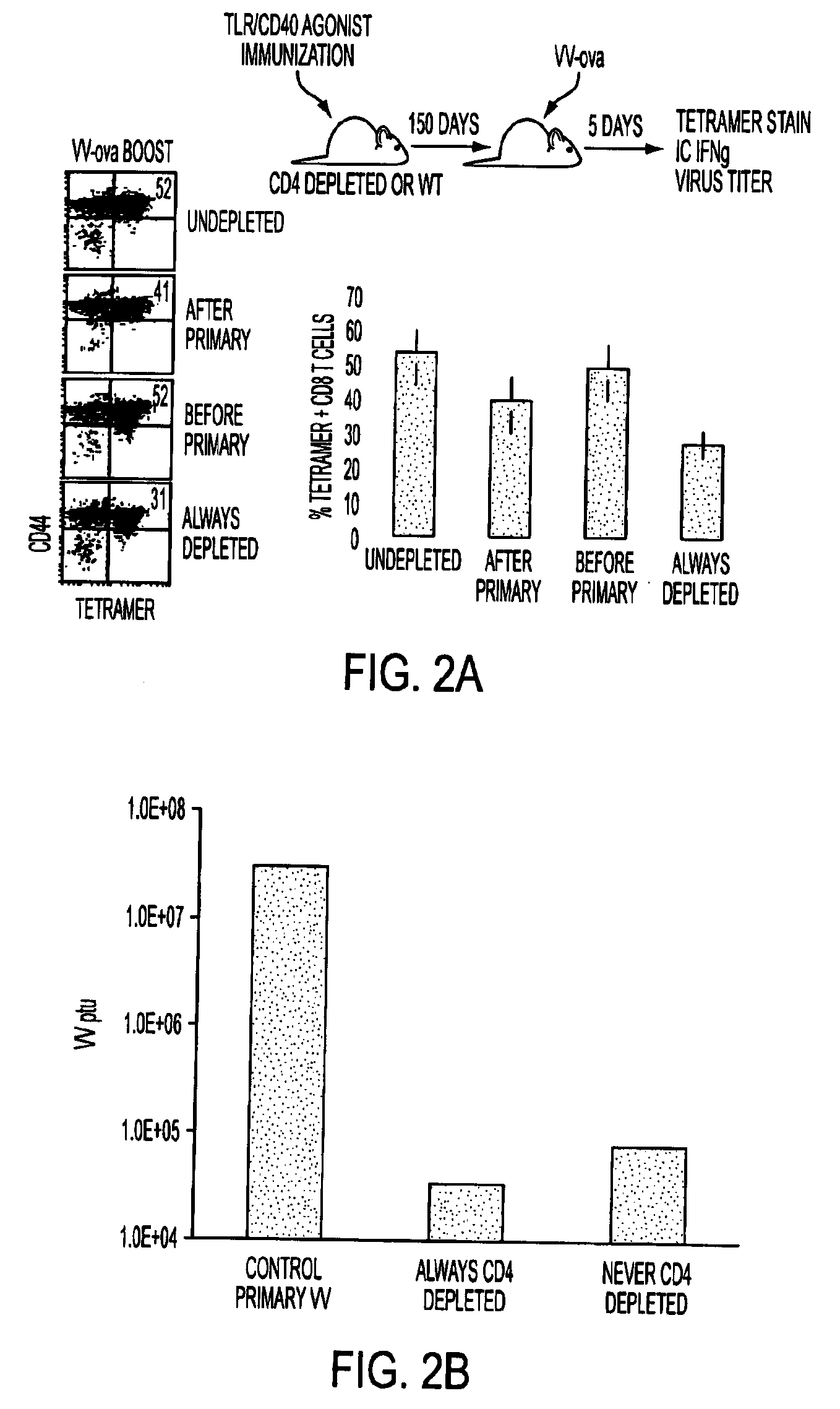
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
InactiveUS20080241139A1Enhanced T cell responseImprove responseAntibacterial agentsAntimycoticsDiseaseYeast
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
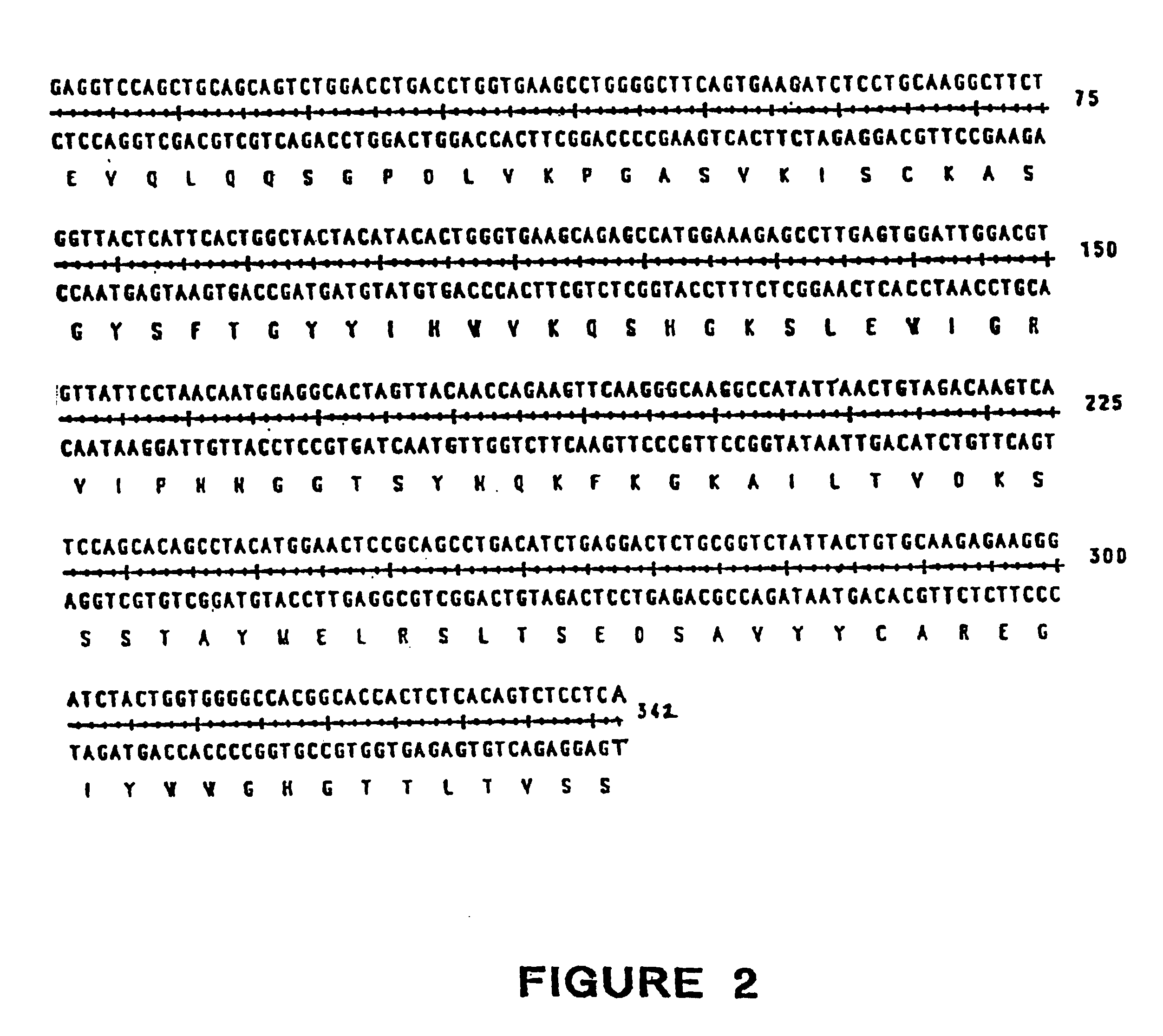
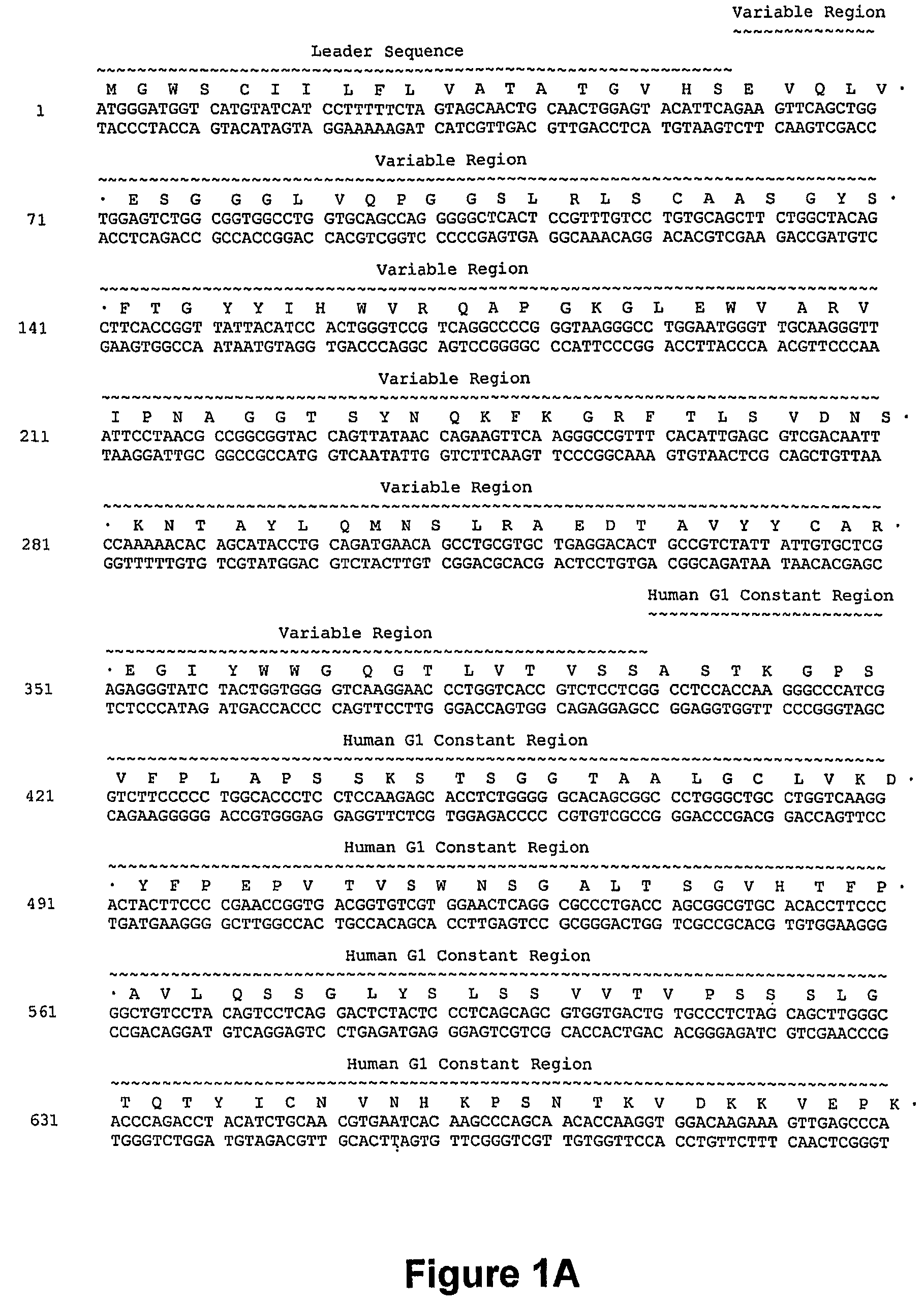
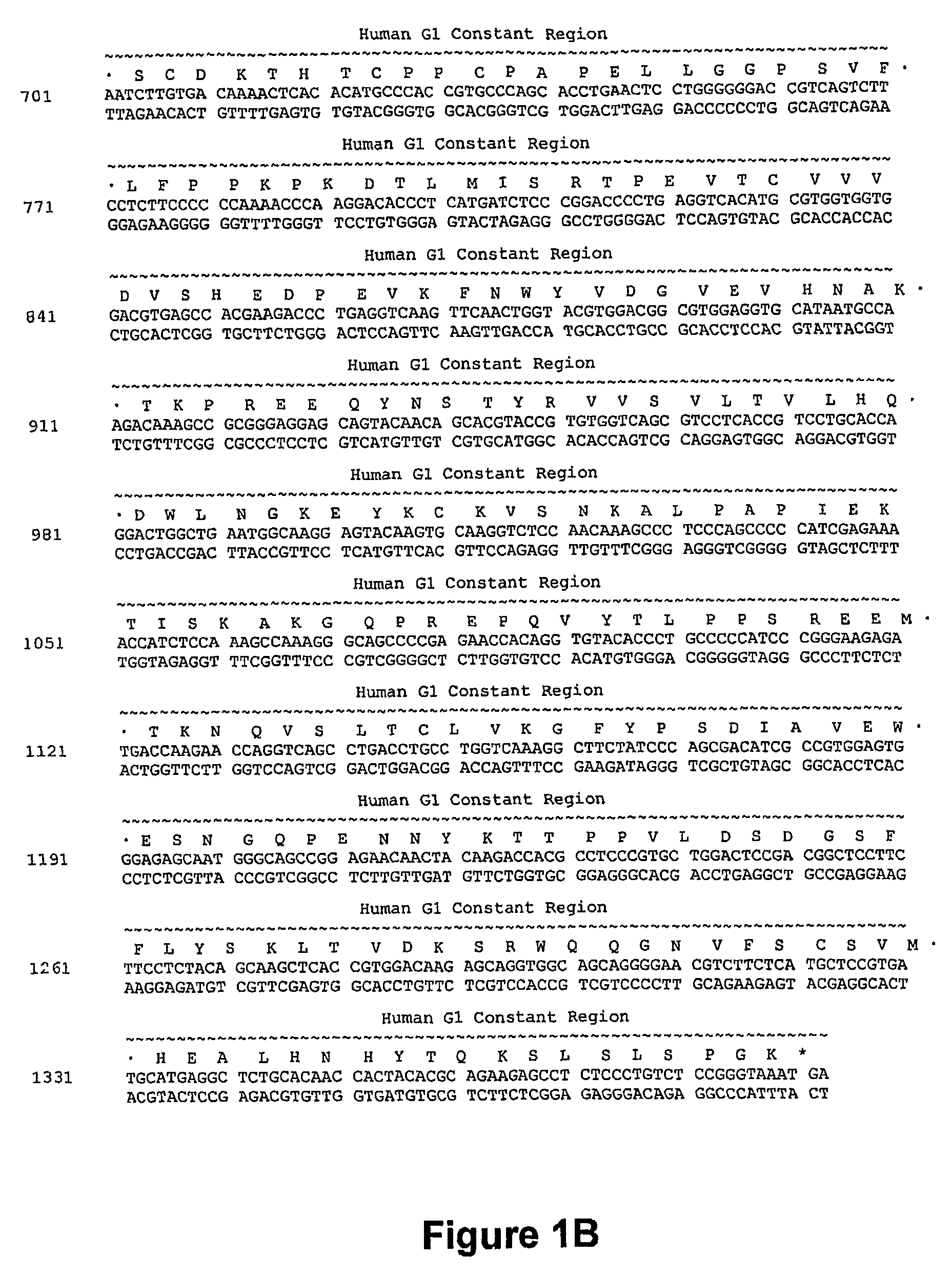
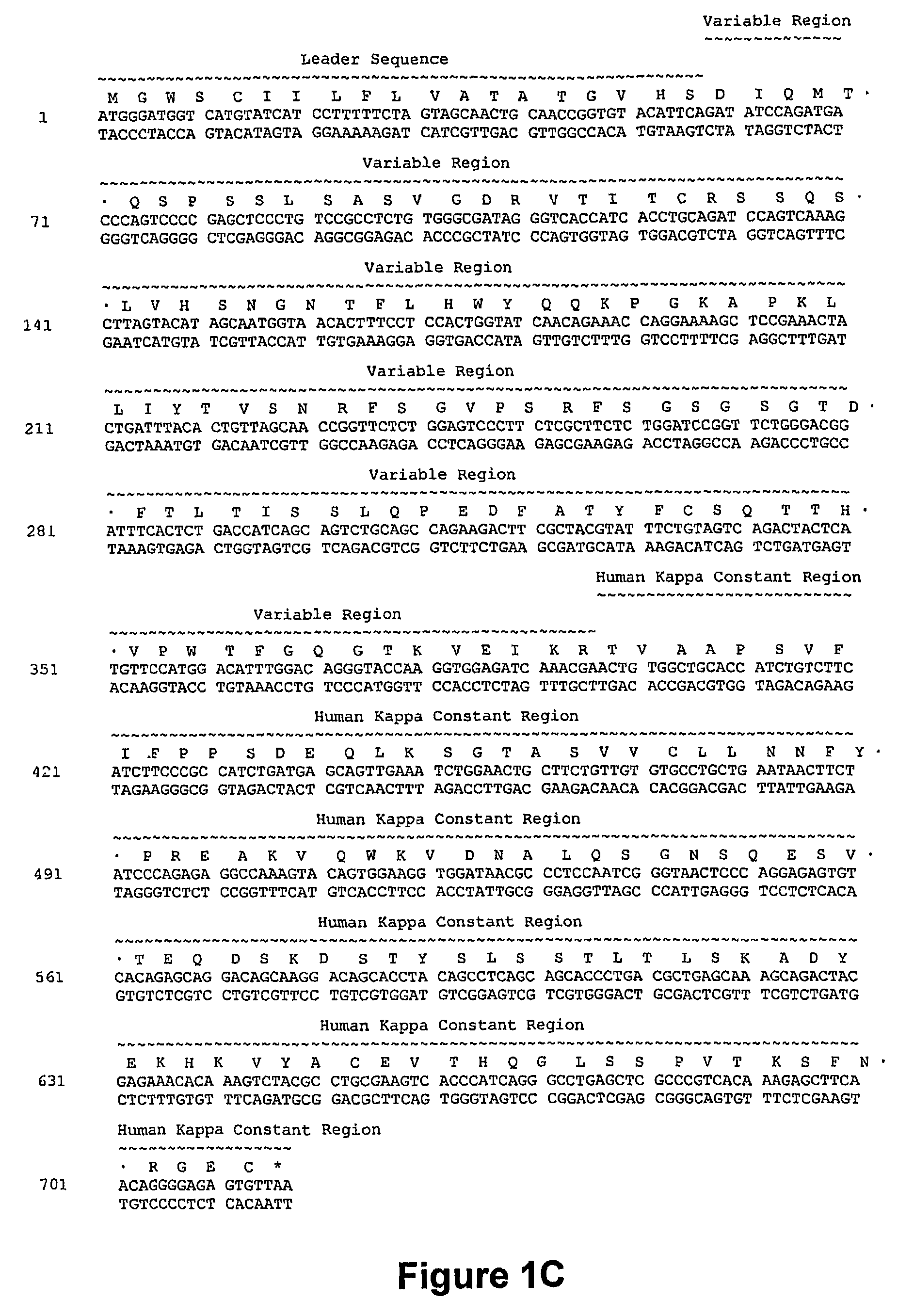
Nucleic acids encoding anti-CD40 proteins and methods of producing recombinant anti-CD40 proteins
The present invention relates to methods and compositions for the prevention and treatment of cancer, inflammatory diseases and disorders or deficiencies of the immune system. The methods of the invention comprise administering a CD40 binding protein that potentiates the binding of CD40 to CD40 ligand.
Owner:SEAGEN INC
Inhibiting B cell activation with soluble CD40 or fusion proteins thereof
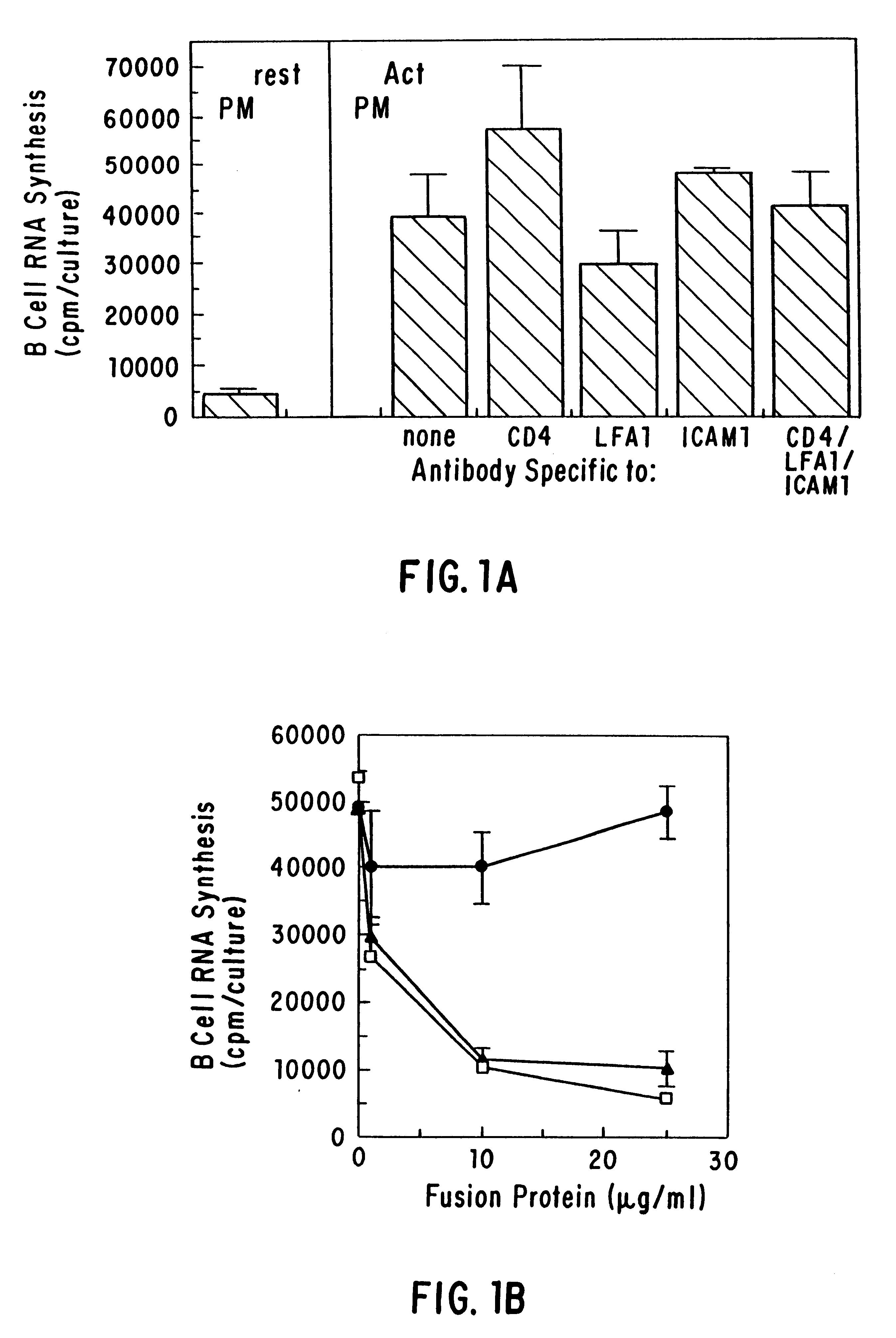
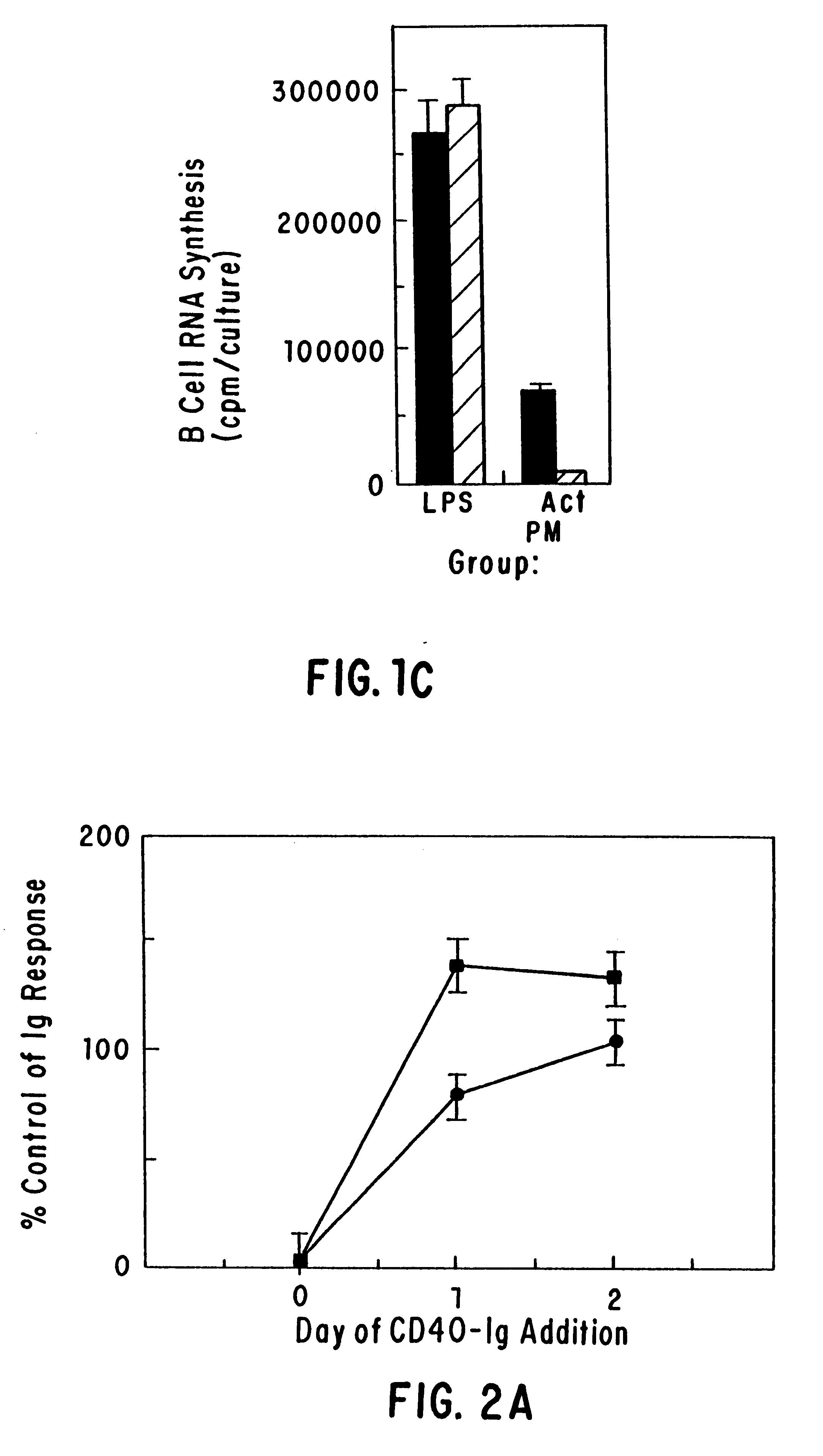
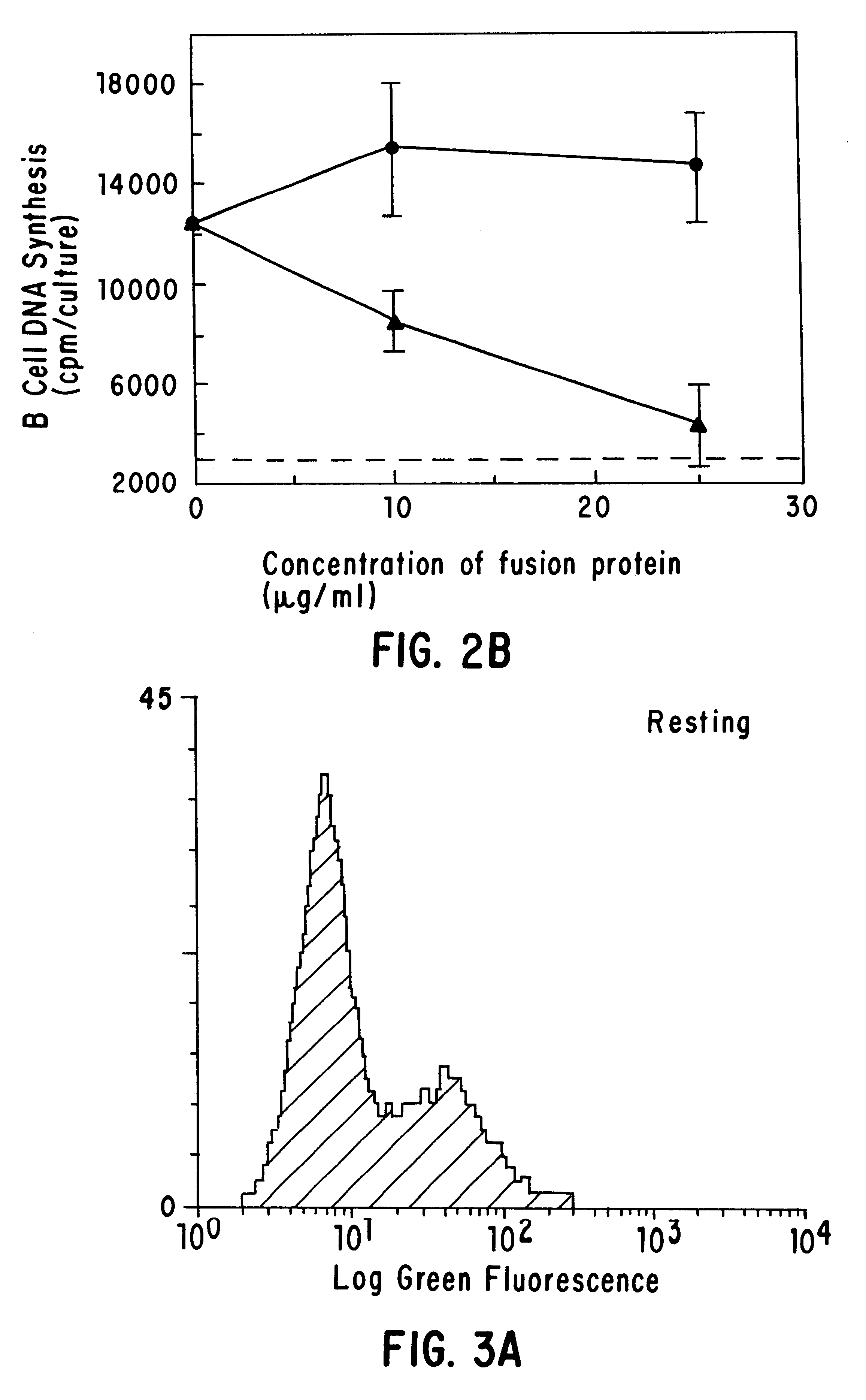
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein or antibody specific for gp39 on T cells was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease, including graft-versus-host disease and rheumatoid arthritis.
Owner:BRISTOL MYERS SQUIBB CO +1
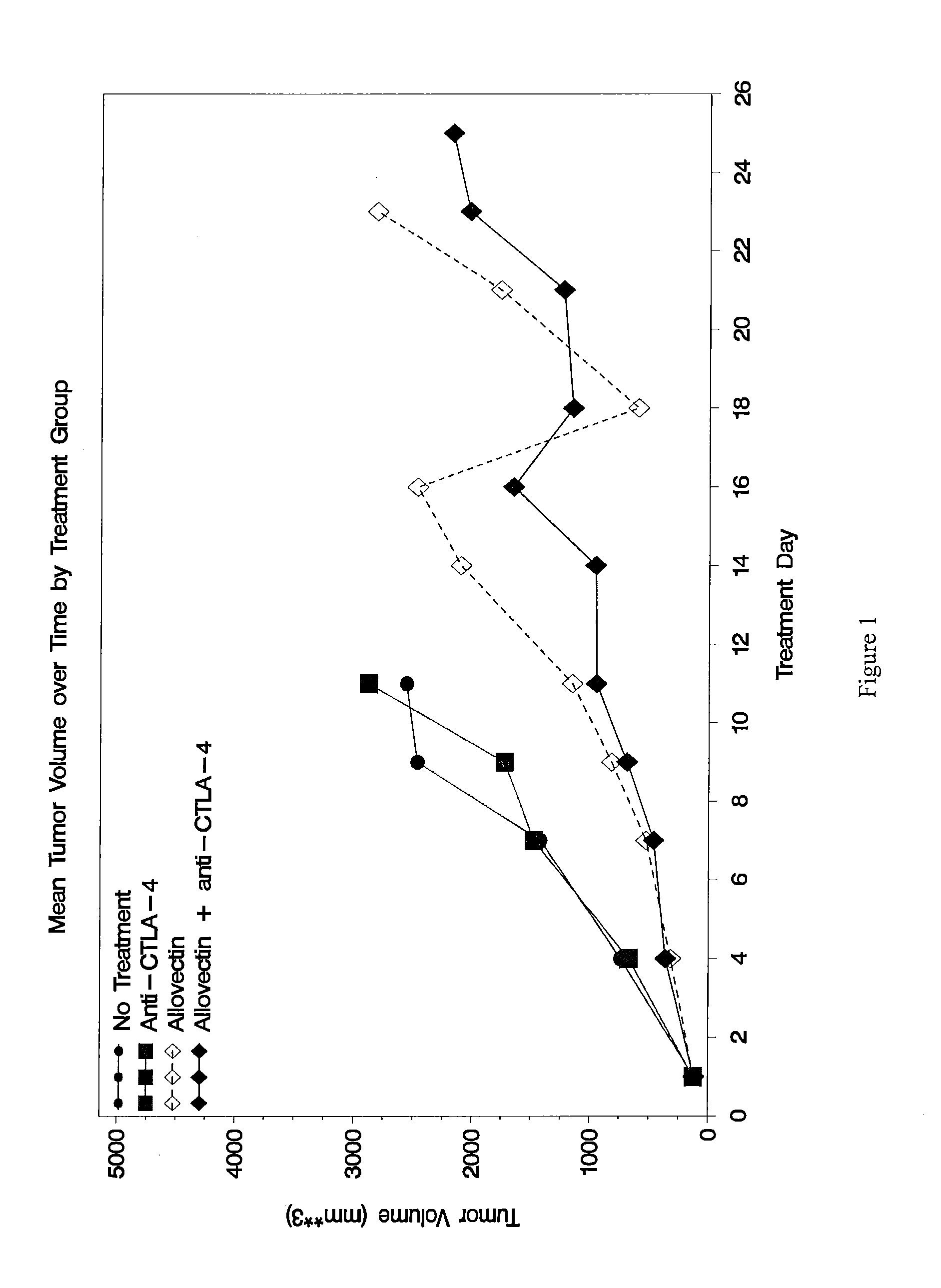
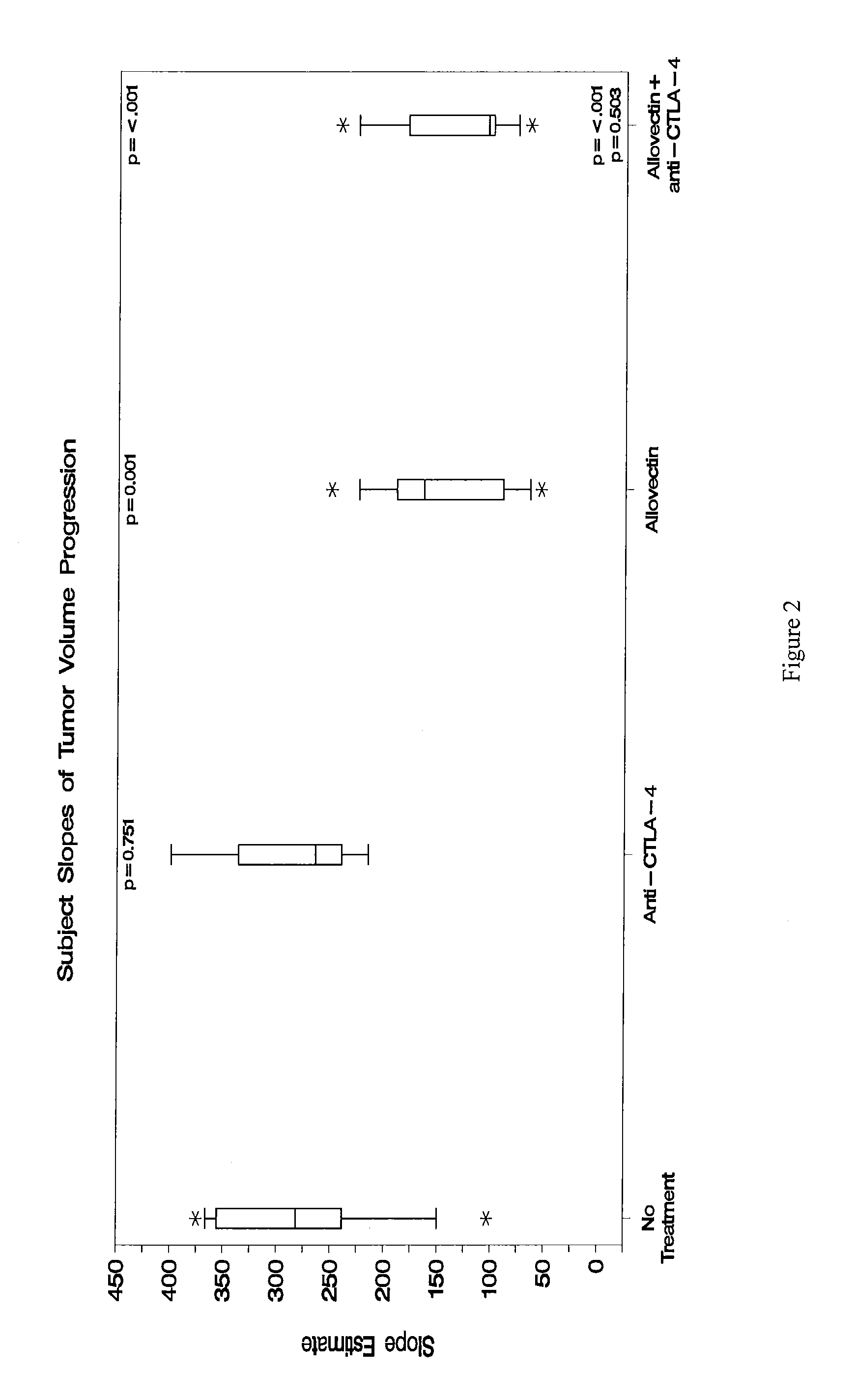
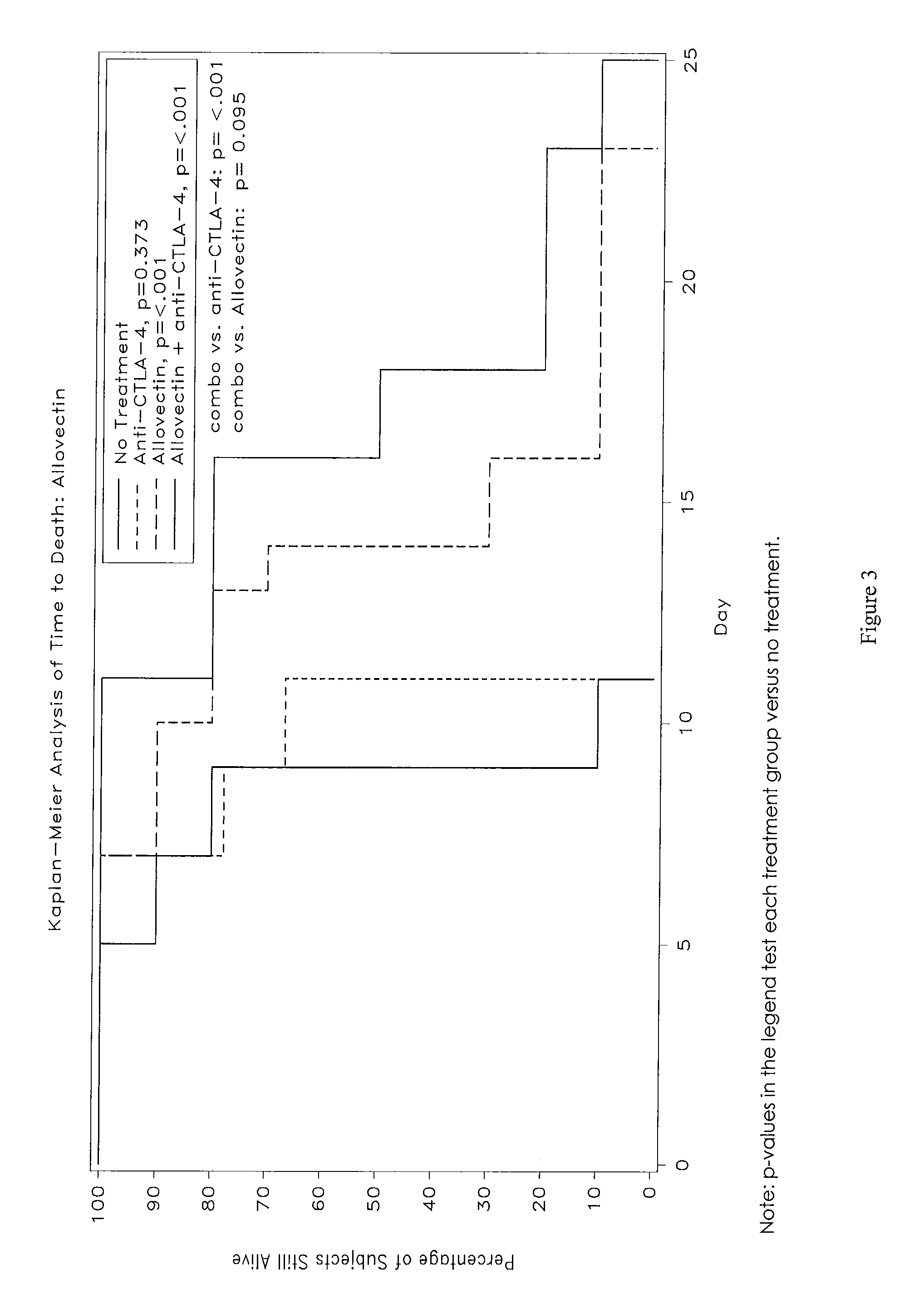
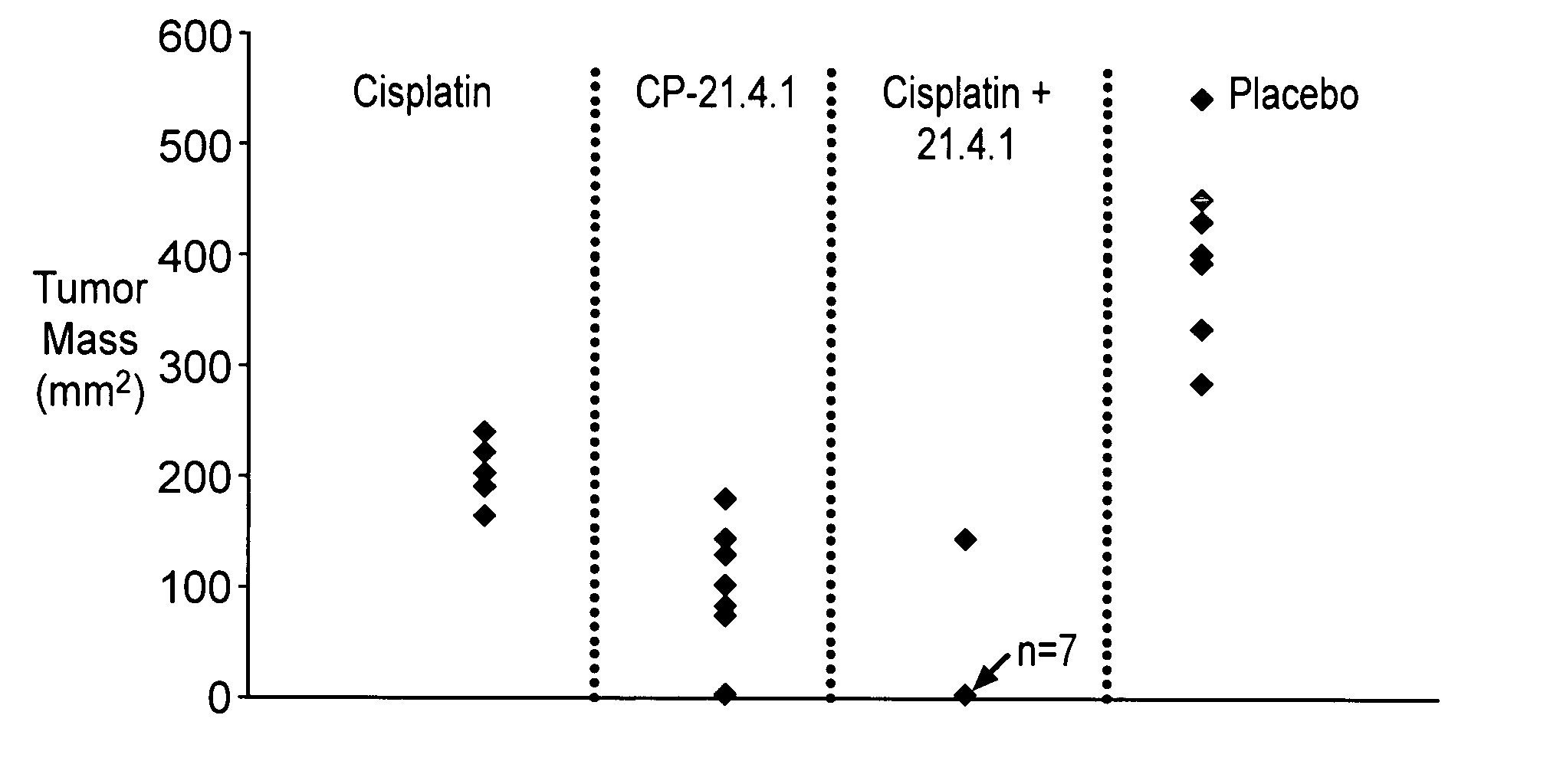
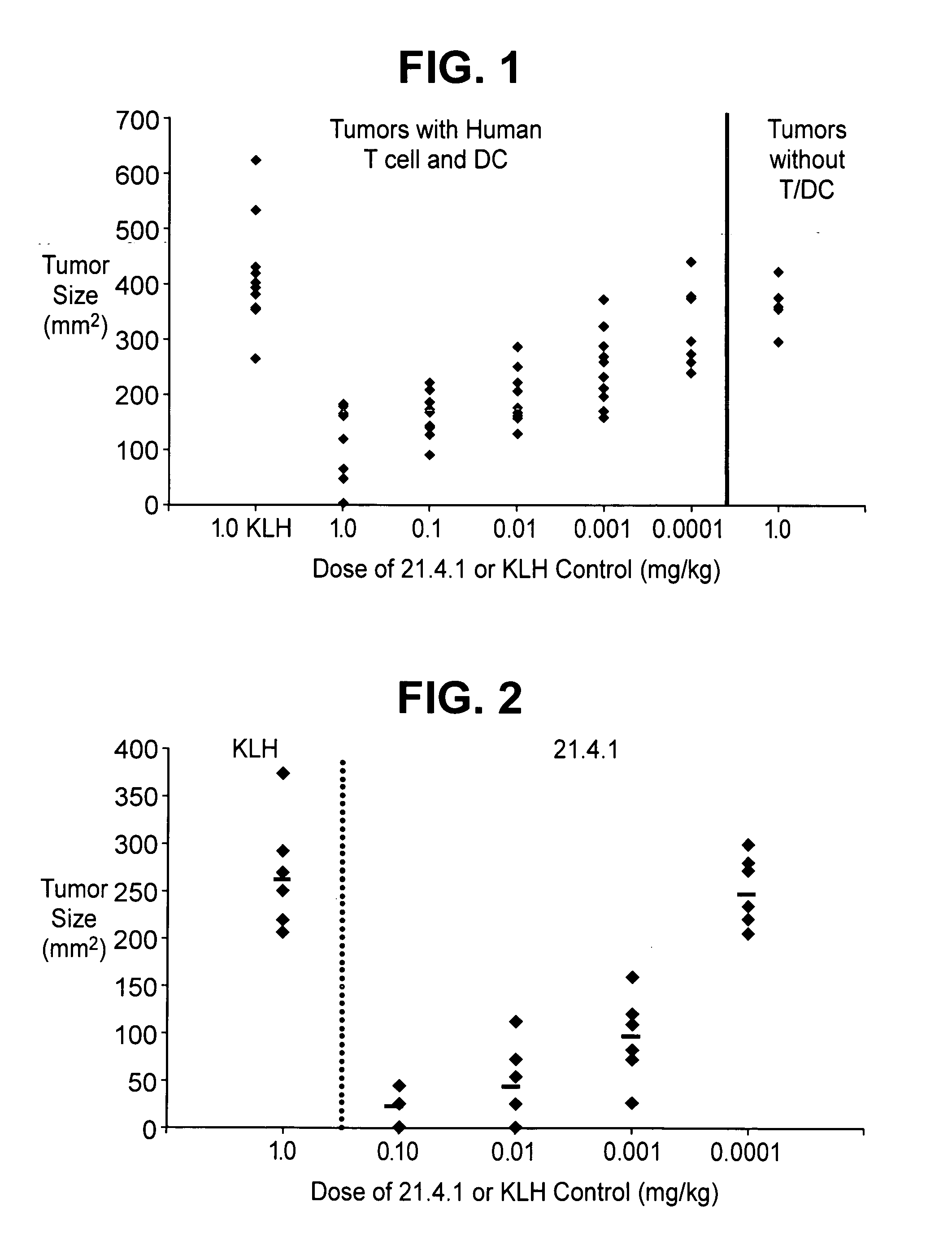
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130280265A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentEfficacy
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
Methods of therapy for B-cell malignancies using antagonist anti-CD40 antibodies
InactiveUS7288252B2Little or no agonist activityNervous disorderVirusesAntigenAntigen Binding Fragment
Methods of therapy for B-cell malignancies are provided. The methods comprise administering a therapeutically effective amount of an antagonist anti-CD40 antibody or antigen-binding fragment thereof to a patient in need thereof. The antagonist anti-CD40 antibody or antigen-binding fragment thereof is free of significant agonist activity when the antibody binds a CD40 antigen on a normal human B cell, exhibits antagonist activity when the antibody binds a CD40 antigen on a malignant human B cell, and can exhibit antagonist activity when the antibody binds a CD40 antigen on a normal human B cell. Antagonist activity of the anti-CD40 antibody or antigen-binding fragment thereof beneficially inhibits proliferation and / or differentiation of malignant human B cells.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
CD86 Antagonist Multi-Target Binding Proteins
This disclosure provides a multi-specific fusion protein composed of a CD86 antagonist binding domain and another binding domain that is an IL-10 agonist, an HLA-G agonist, an HGF agonist, an IL-35 agonist, a PD-1 agonist, a BTLA agonist, a LIGHT antagonist, a GITRL antagonist or a CD40 antagonist. The multi-specific fusion protein may also include an intervening domain that separates the other domains. This disclosure also provides polynucleotides encoding the multi-specific fusion proteins, compositions of the fusion proteins, and methods of using the multi-specific fusion proteins and compositions.
Owner:APTEVO RES & DEV LLC
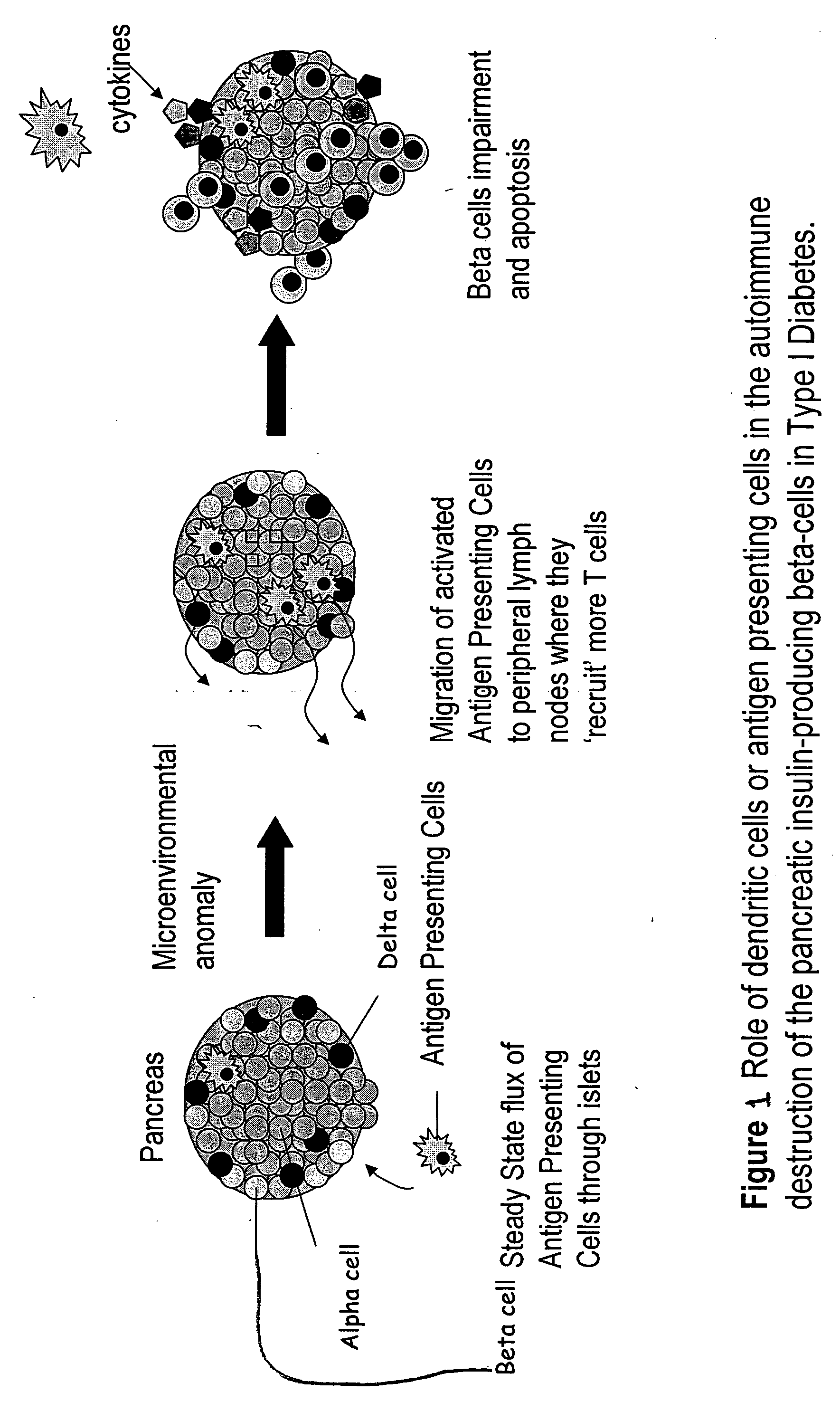
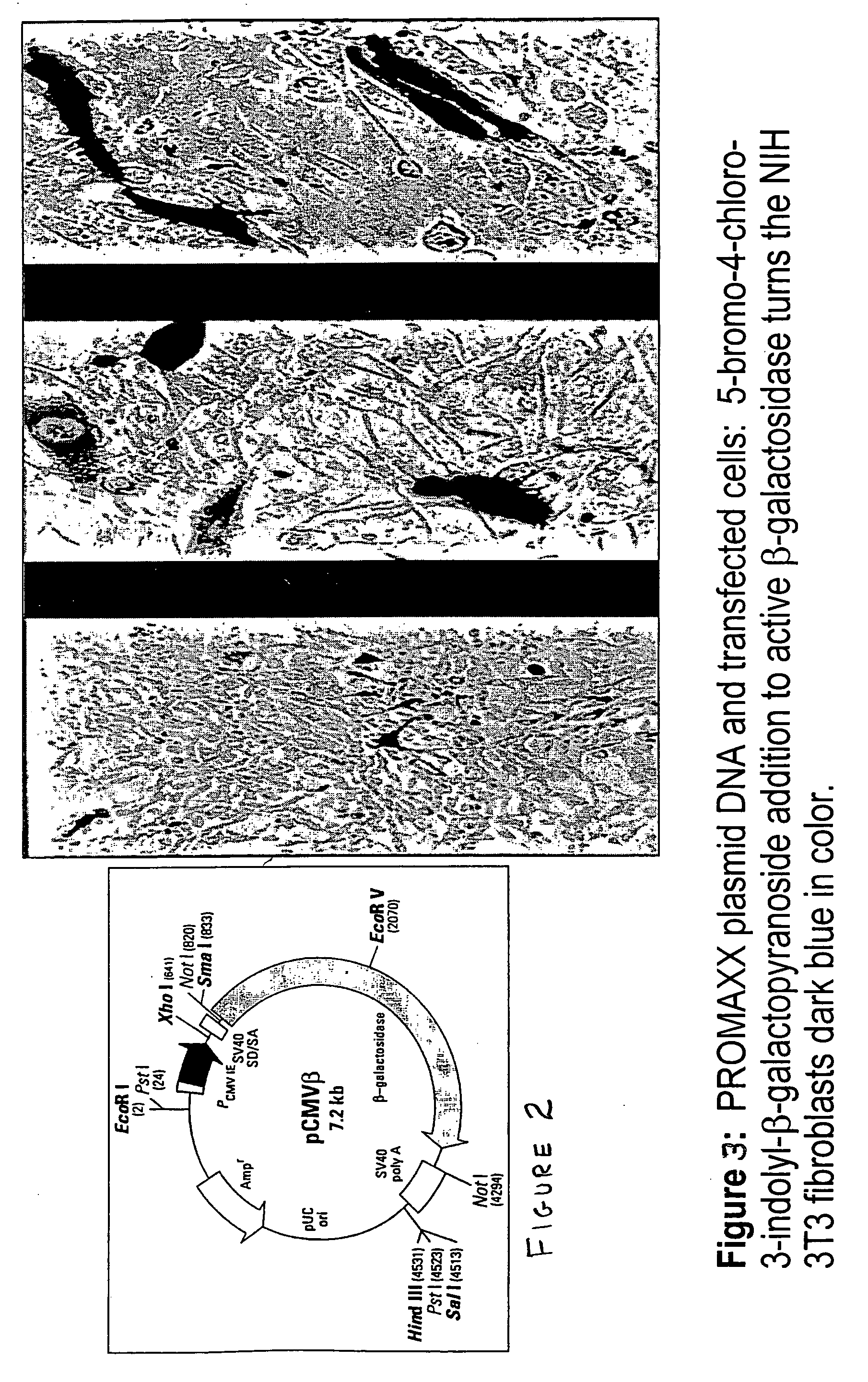
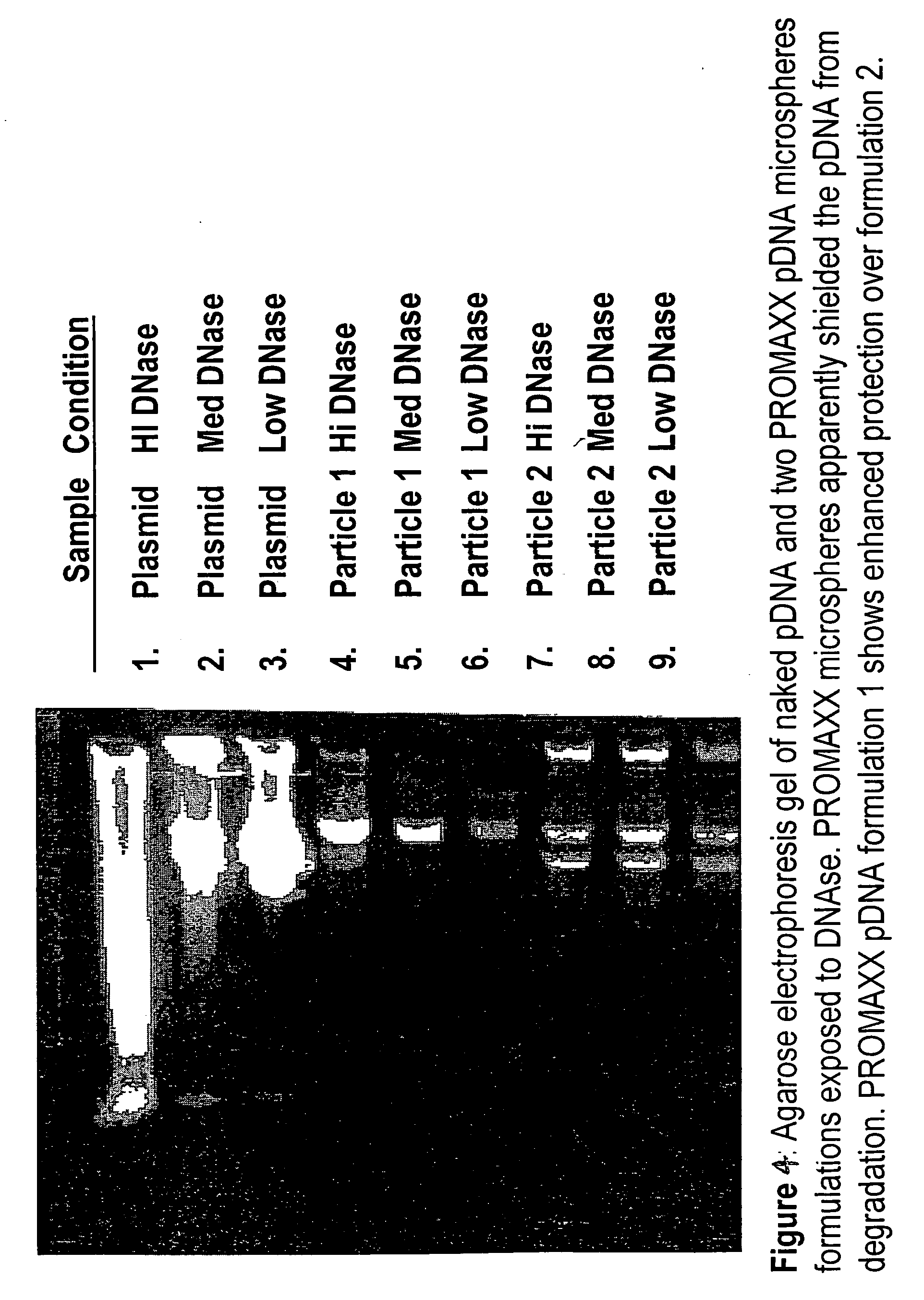
Delivery of as-oligonucleotide microspheres to induce dendritic cell tolerance for the treatment of autoimmune type 1 diabetes
AS-oligonucleotides are delivered in microsphere form in order to induce dendritic cell tolerance, particularly in the non-obese-diabetic (NOD) mouse model. The microspheres incorporate antisense (AS) oligonucleotides. A process includes using an antisense approach to prevent an autoimmune diabetes condition in NOD mice in vivo and in situ. The oligonucleotides are targeted to bind to primary transcripts CD40, CD80, CD86 and their combinations.
Owner:BAXTER INT INC +2
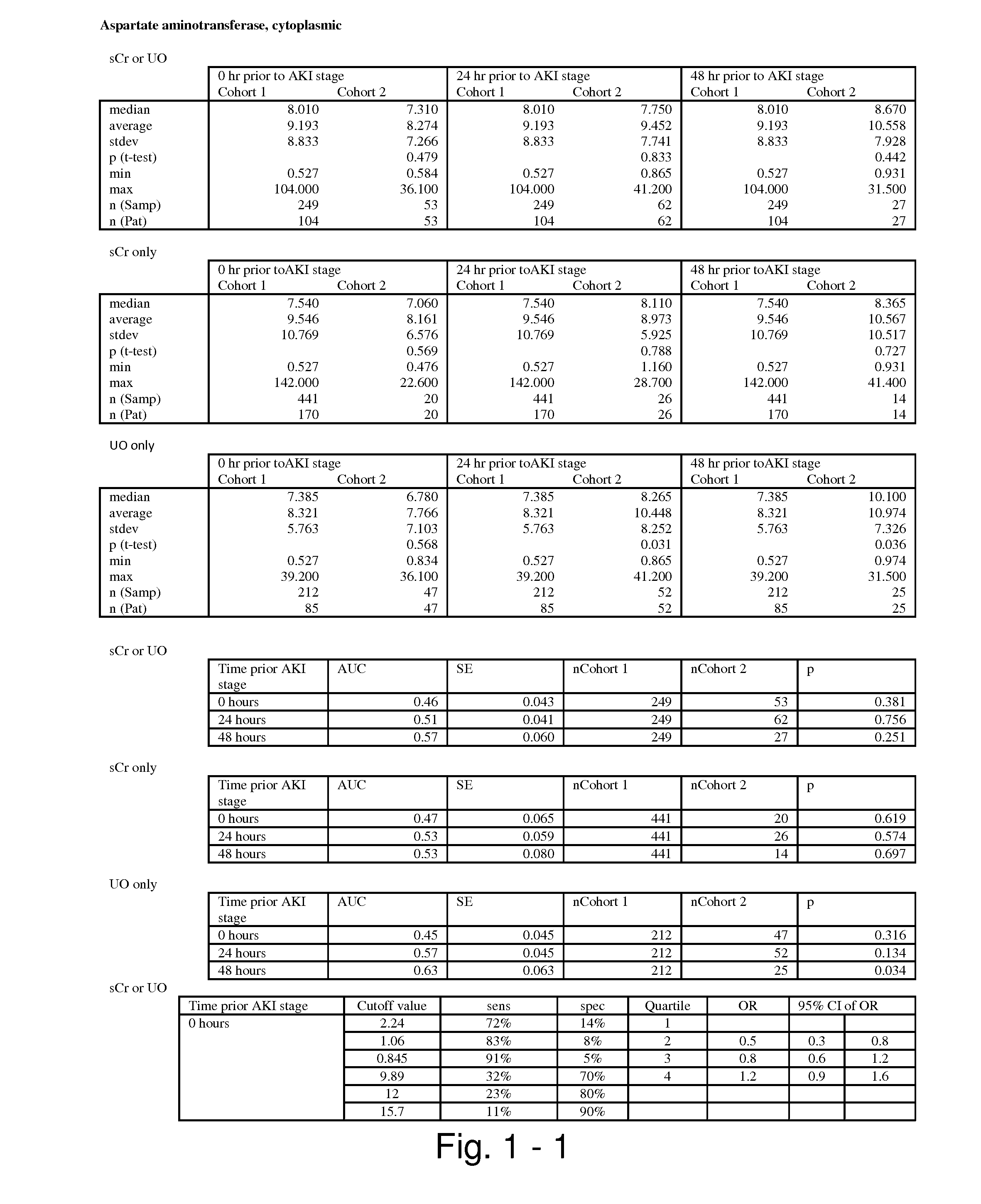
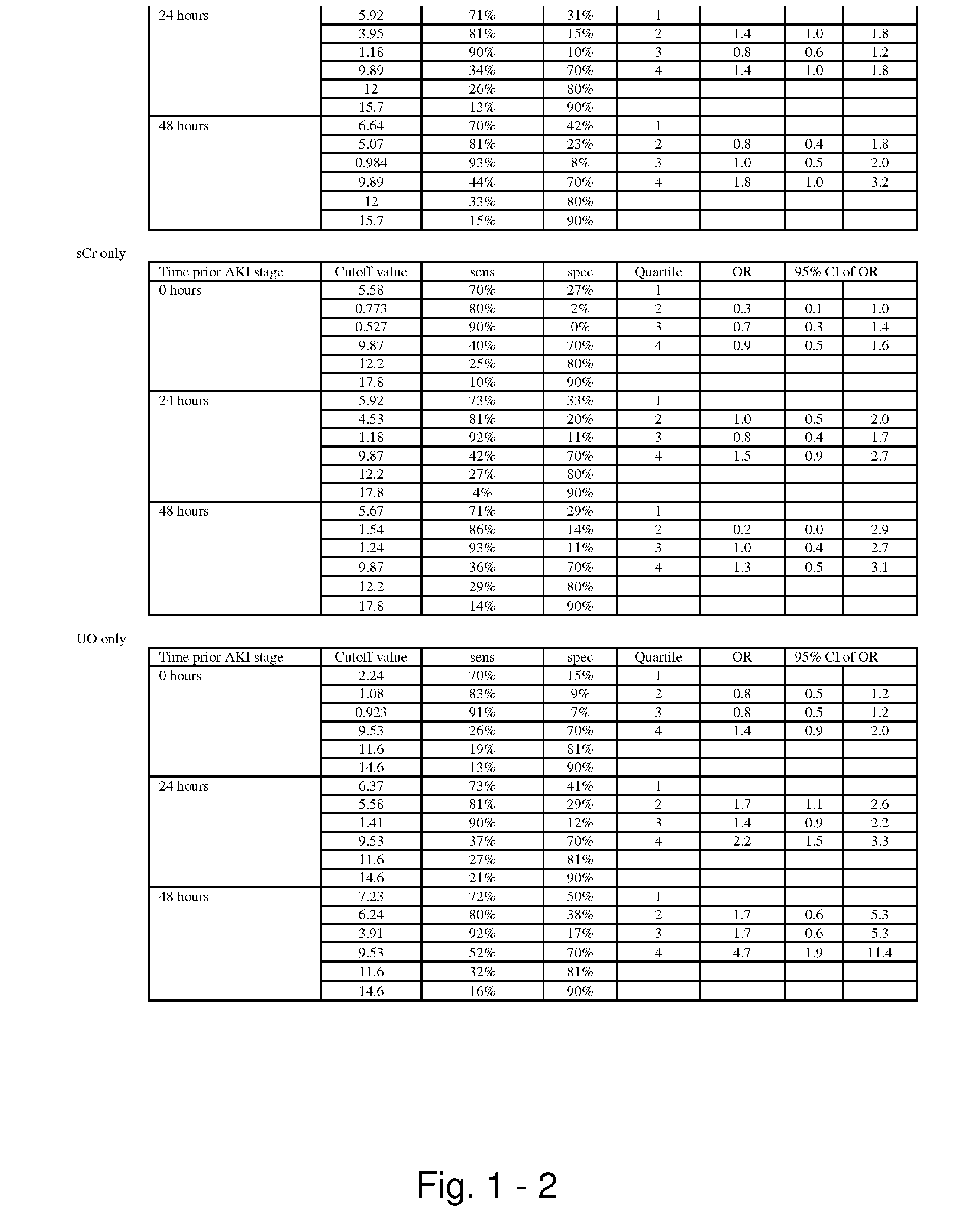
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20110201038A1Easy to adaptMicrobiological testing/measurementDisease diagnosisInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
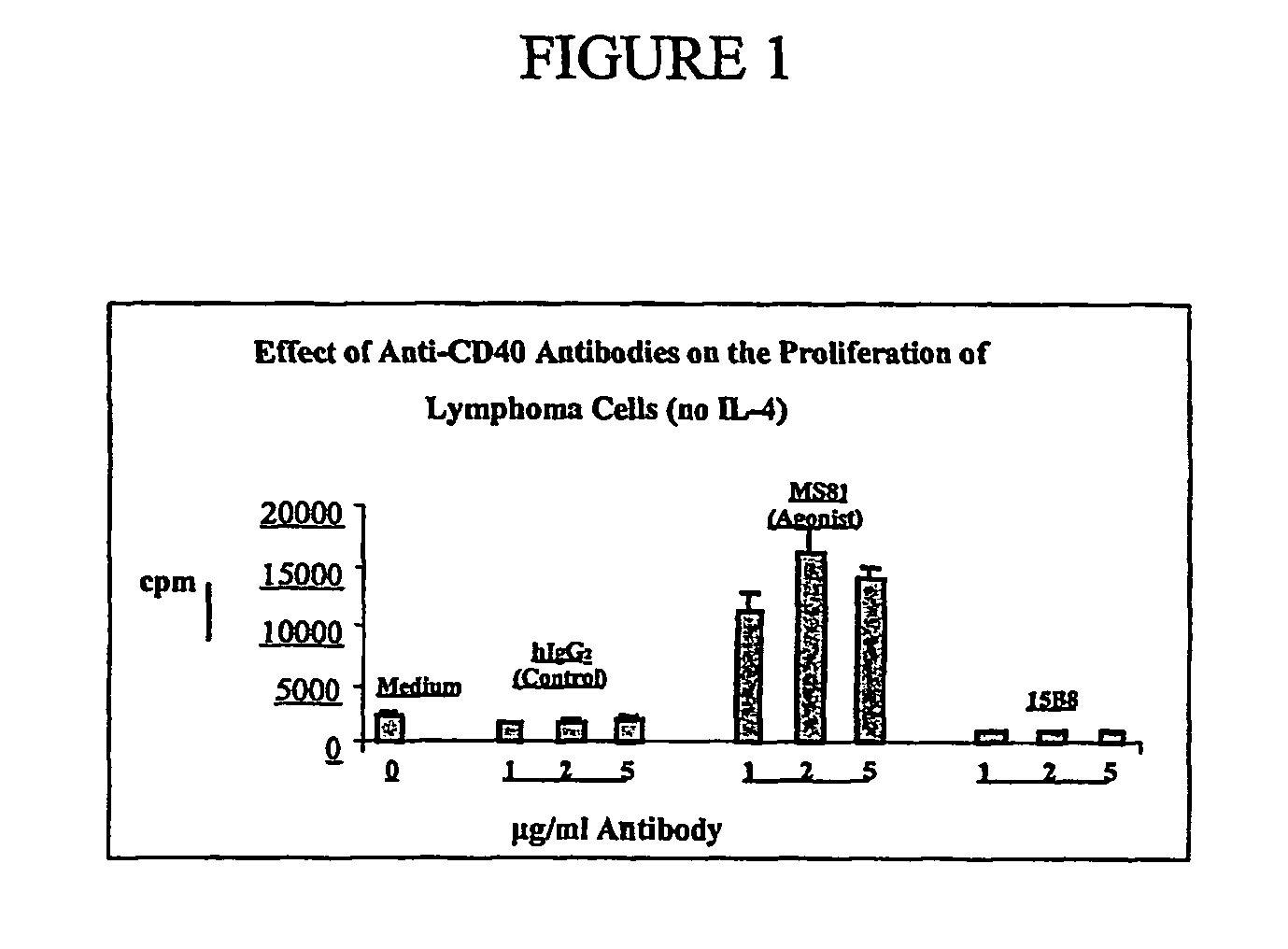
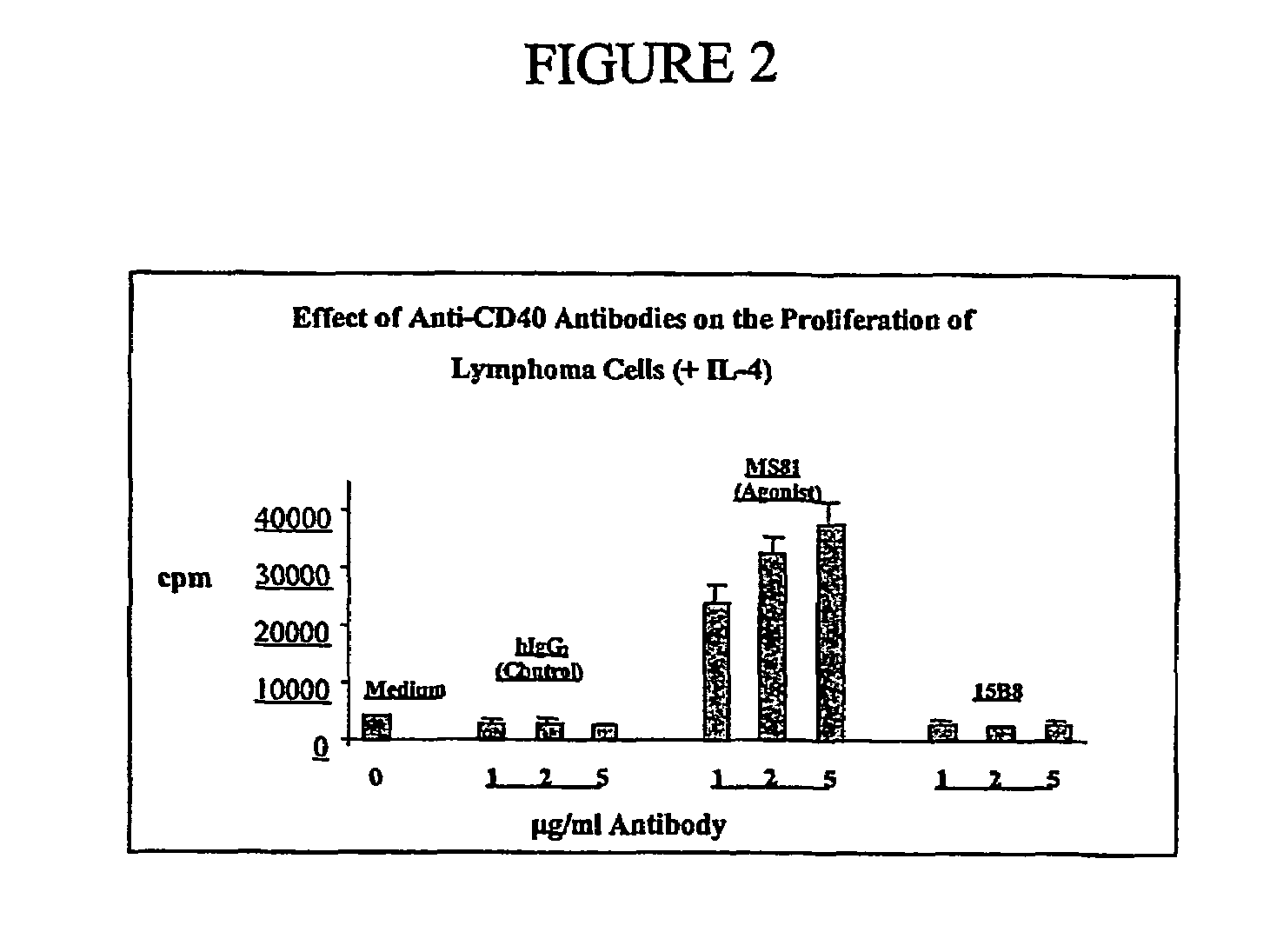
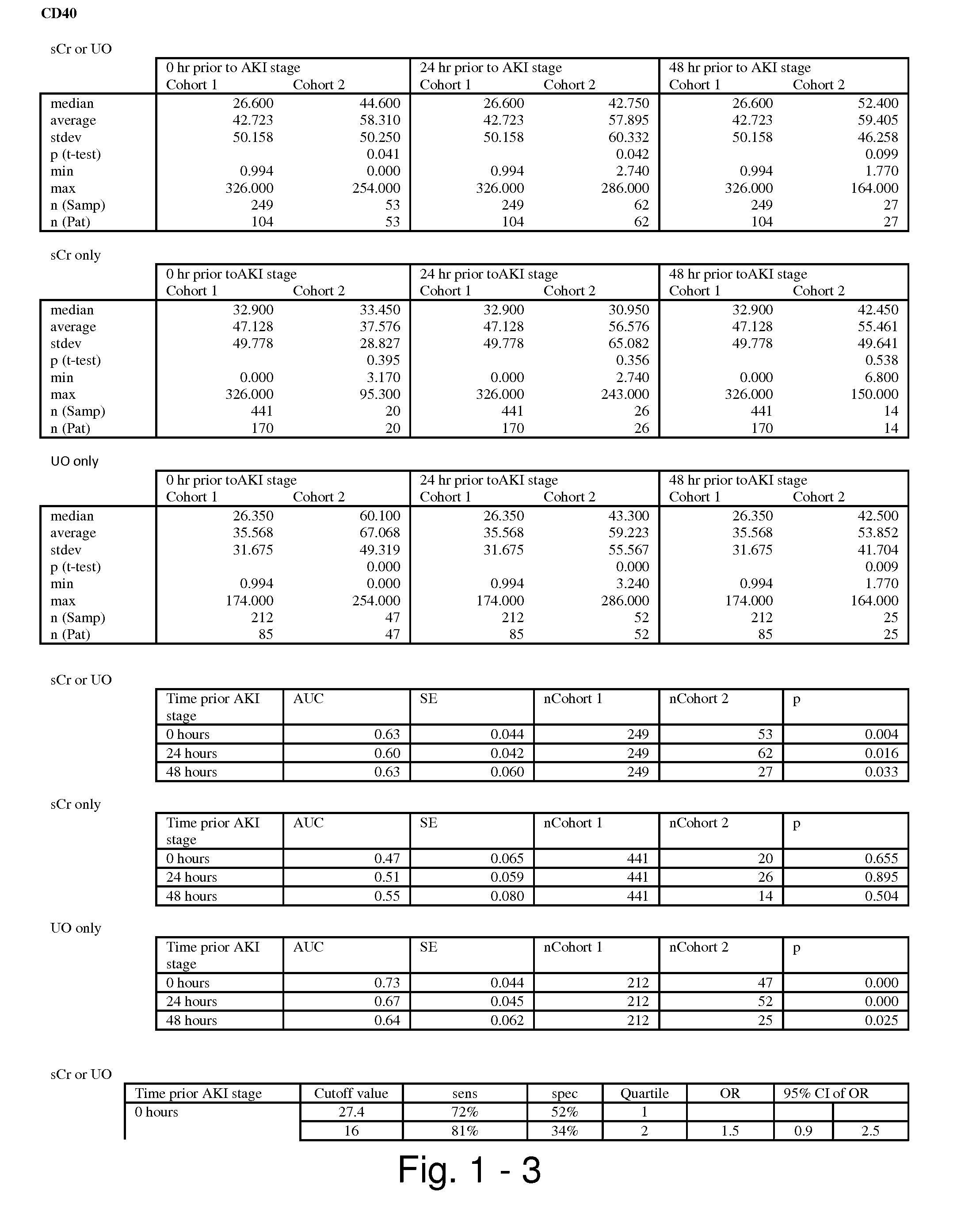
CD40 antibody formulation and methods
InactiveUS20050136055A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesAgonist
The present invention provides a method of treating tumor in a patient comprising administering to said patient a CD40 agonist antibody according to an intermittent dosing schedule. The present invention also provides a method of treating tumor in a patient comprising administering a combination of a CD40 agonist antibody and a DNA replication inhibitor. Also provided is a formulation for use in the treatment.
Owner:PFIZER INC
CD40 receptor ligands
InactiveUS6472510B1Analysis impossiblePeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseCell membrane
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease.
Owner:BRISTOL MYERS SQUIBB CO
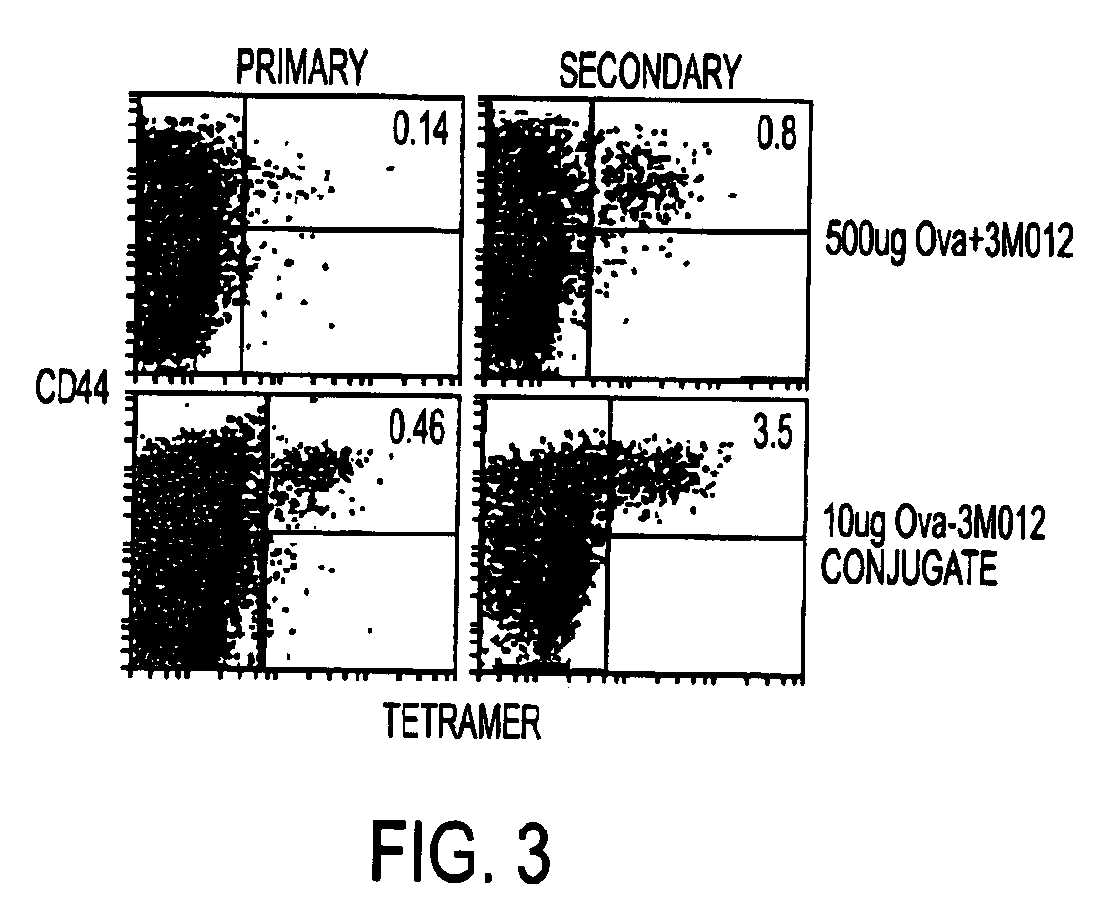
Tlr agonist (flagellin)/cd40 agonist/antigen protein and DNA conjugates and use thereof for inducing synergistic enhancement in immunity
Owner:KEDL ROSS
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20120190044A1Eliminate needBioreactor/fermenter combinationsBiological substance pretreatmentsGlycoproteinMetalloproteinase inhibitor
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a plurality of assays, one or more of which is configured to detect a kidney injury marker selected from the group consisting of metalloproteinase inhibitor 2, soluble oxidized low-density lipoprotein receptor 1, interleukin-2, von Willebrand factor, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor receptor superfamily member 11B, neutrophil elastase, interleukin-1 beta, heart-type fatty acid-binding protein, beta-2-glycoprotein 1, soluble CD40 ligand, coagulation factor VII, C—C motif chemokine 2, IgM, CA 19-9, IL-10, TNF-α, and myoglobin as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Methods of using cd40 binding agents
InactiveUS20090304687A1Enhanced interactionActivityAntibody ingredientsImmunoglobulinsDiseaseImmunology
Owner:SEATTLE GENETICS INC
Adenoviral expression vector comprising a CD40L fusion protein adapted to elicit cellular immunity
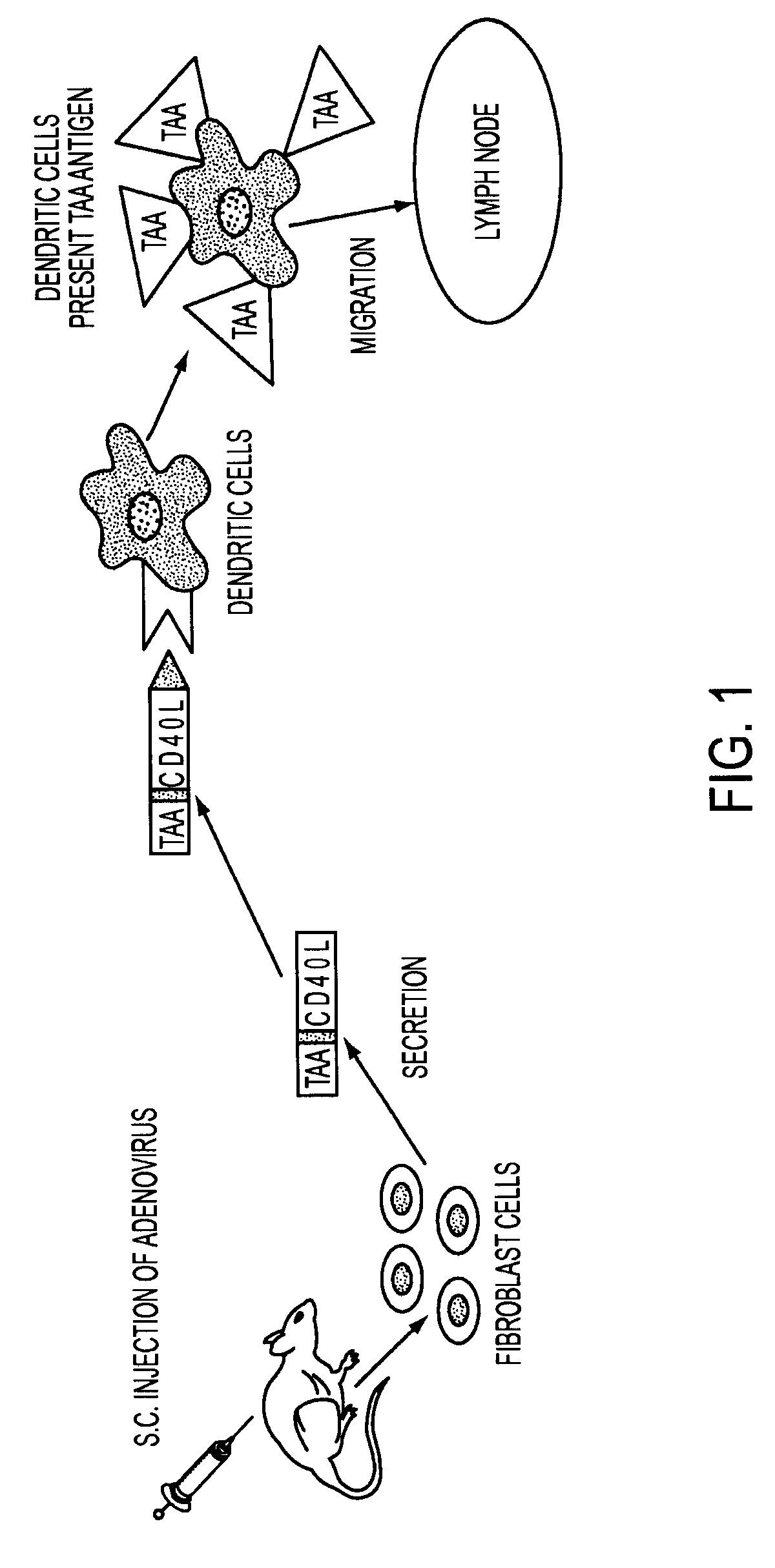
ActiveUS8119117B2Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsTransmembrane domainTumor antigen
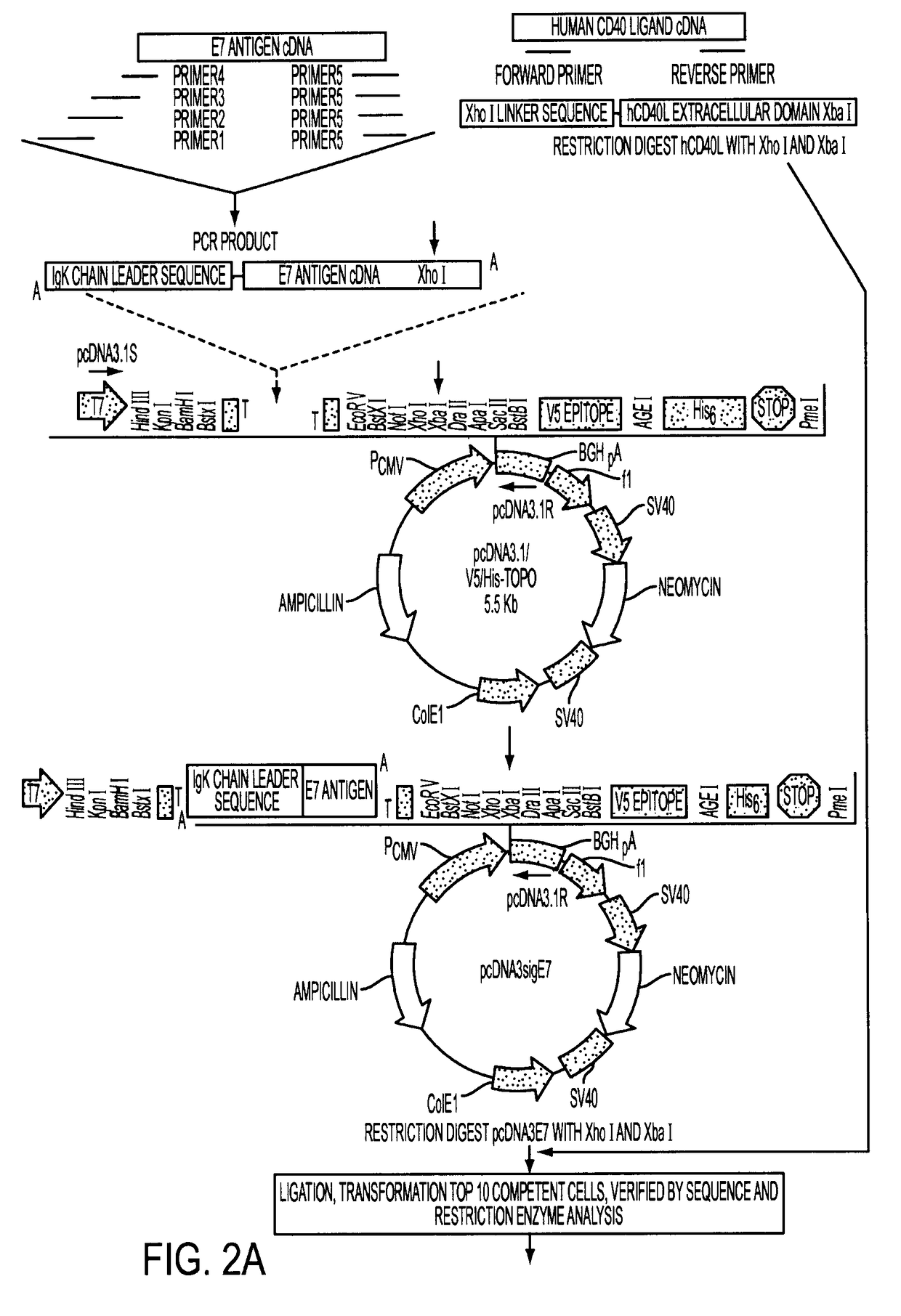
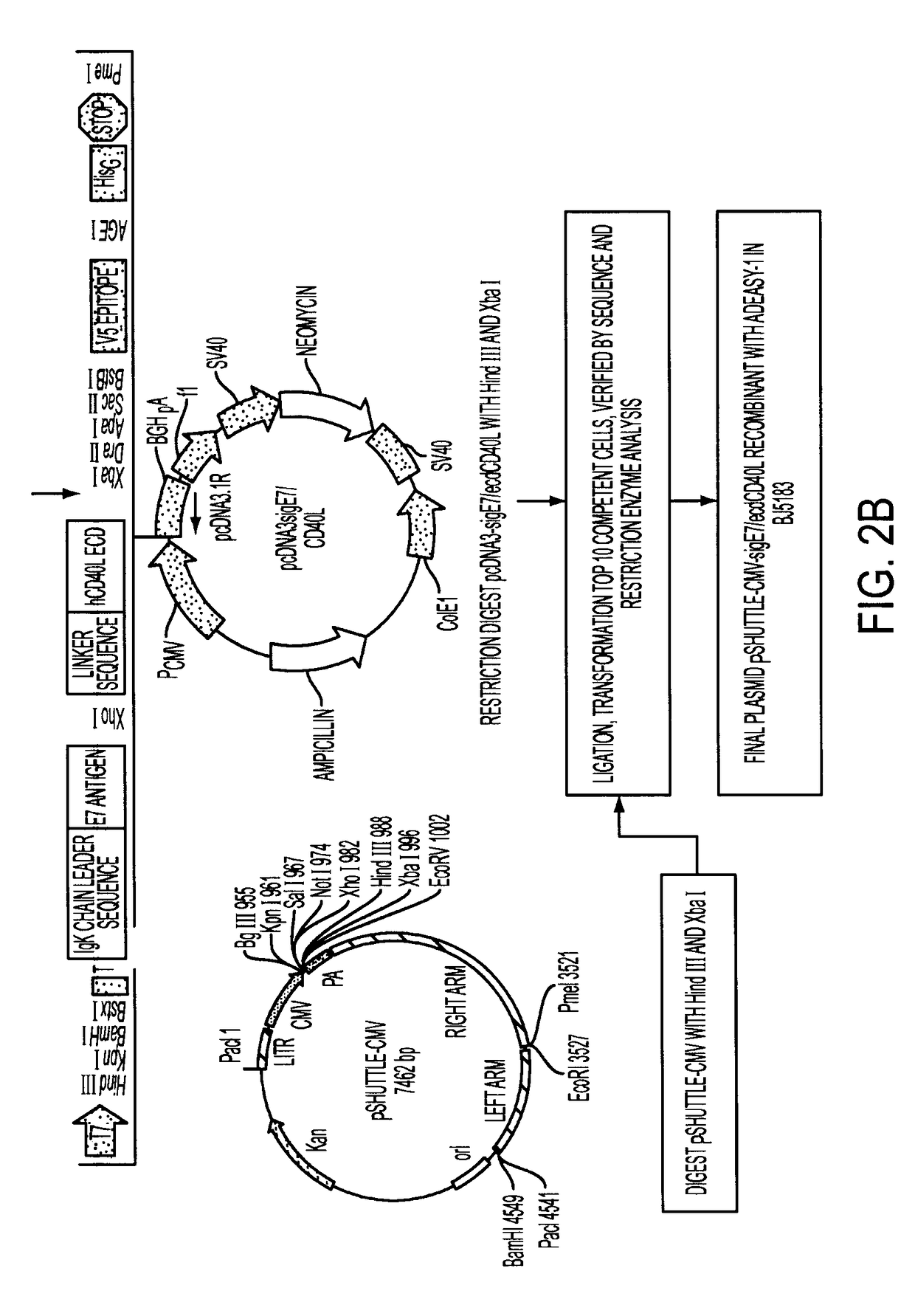
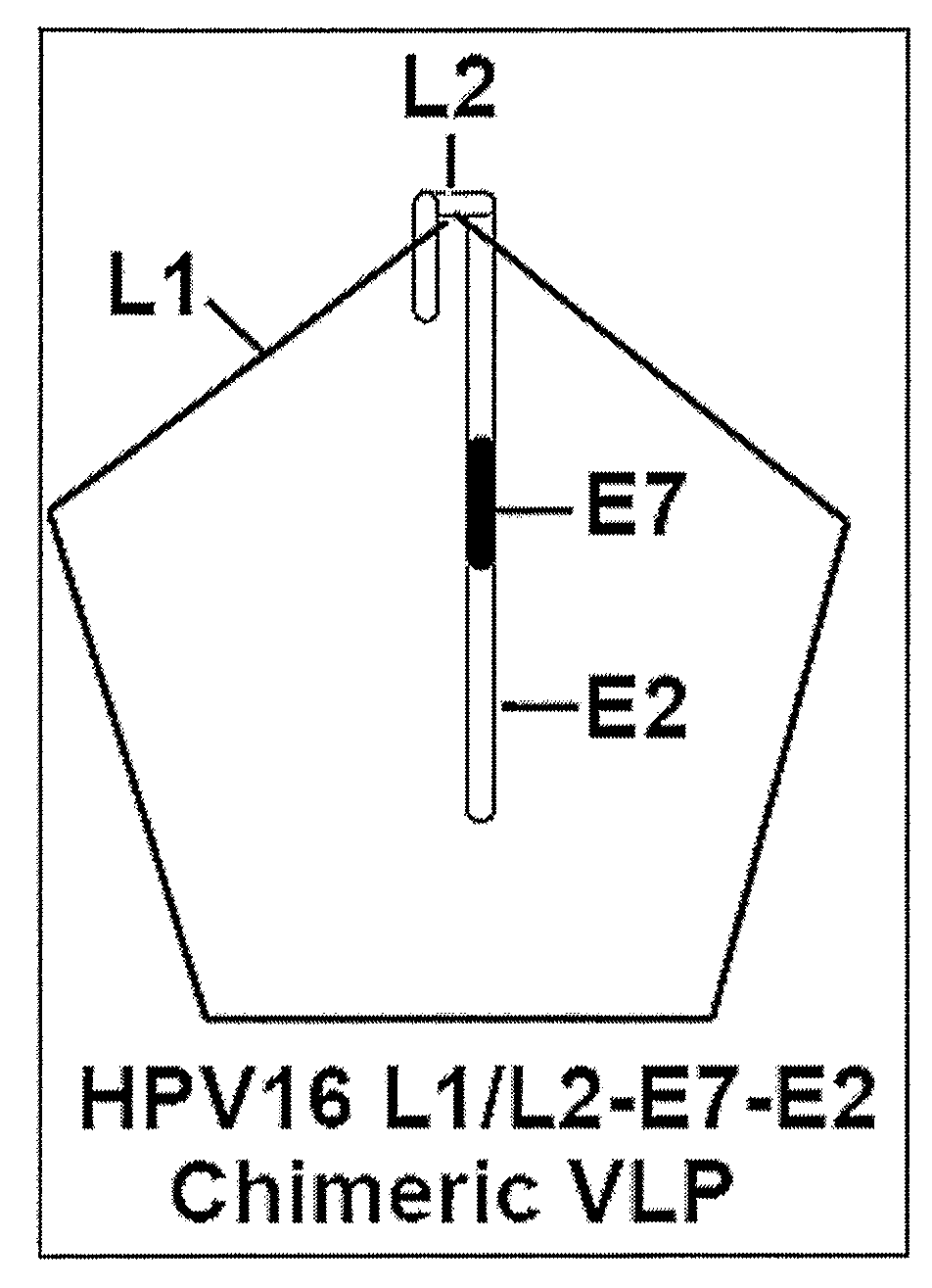
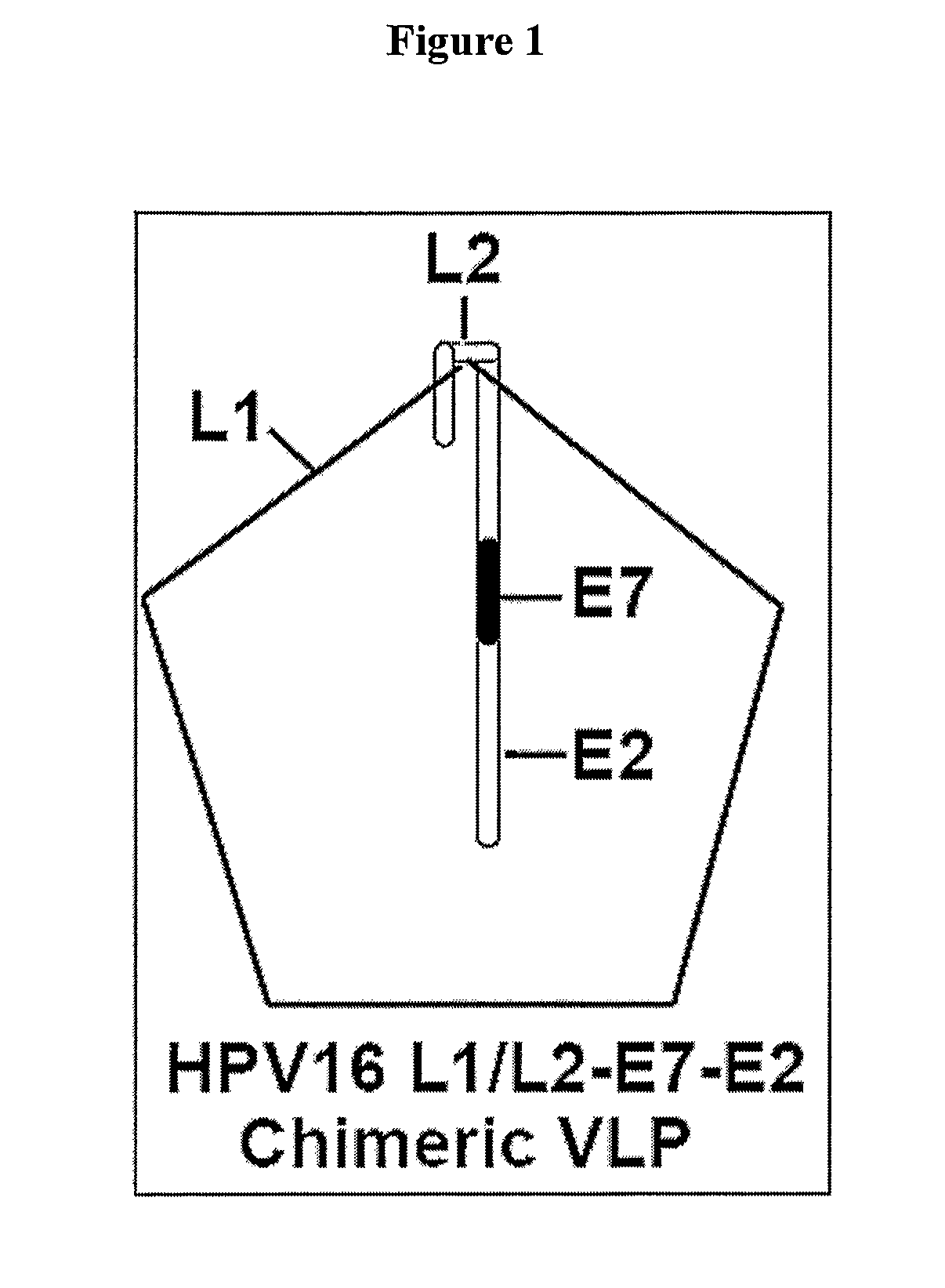
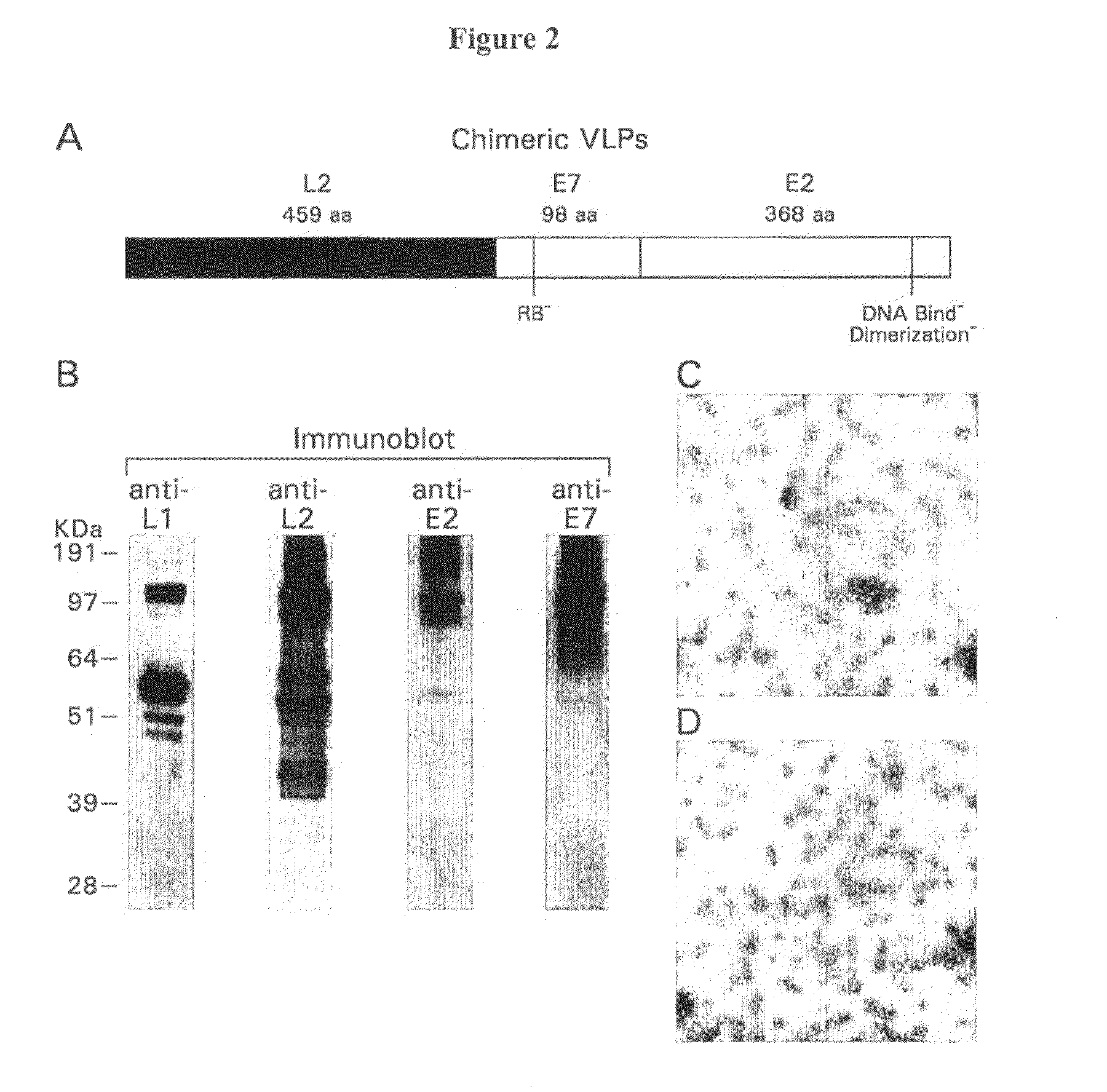
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
CD40 splice variants and their uses
InactiveUS20050281815A1Improve accuracyHigh detection sensitivityAnimal cellsBacteriaMedicineGenetics
Disclosed are CD40 splice variant polypeptides and methods of using the CD40 splice variant polypeptides, including in treatment for transplantation.
Owner:ESHEL DANI +3
Uses of Anti-cd40 antibodies
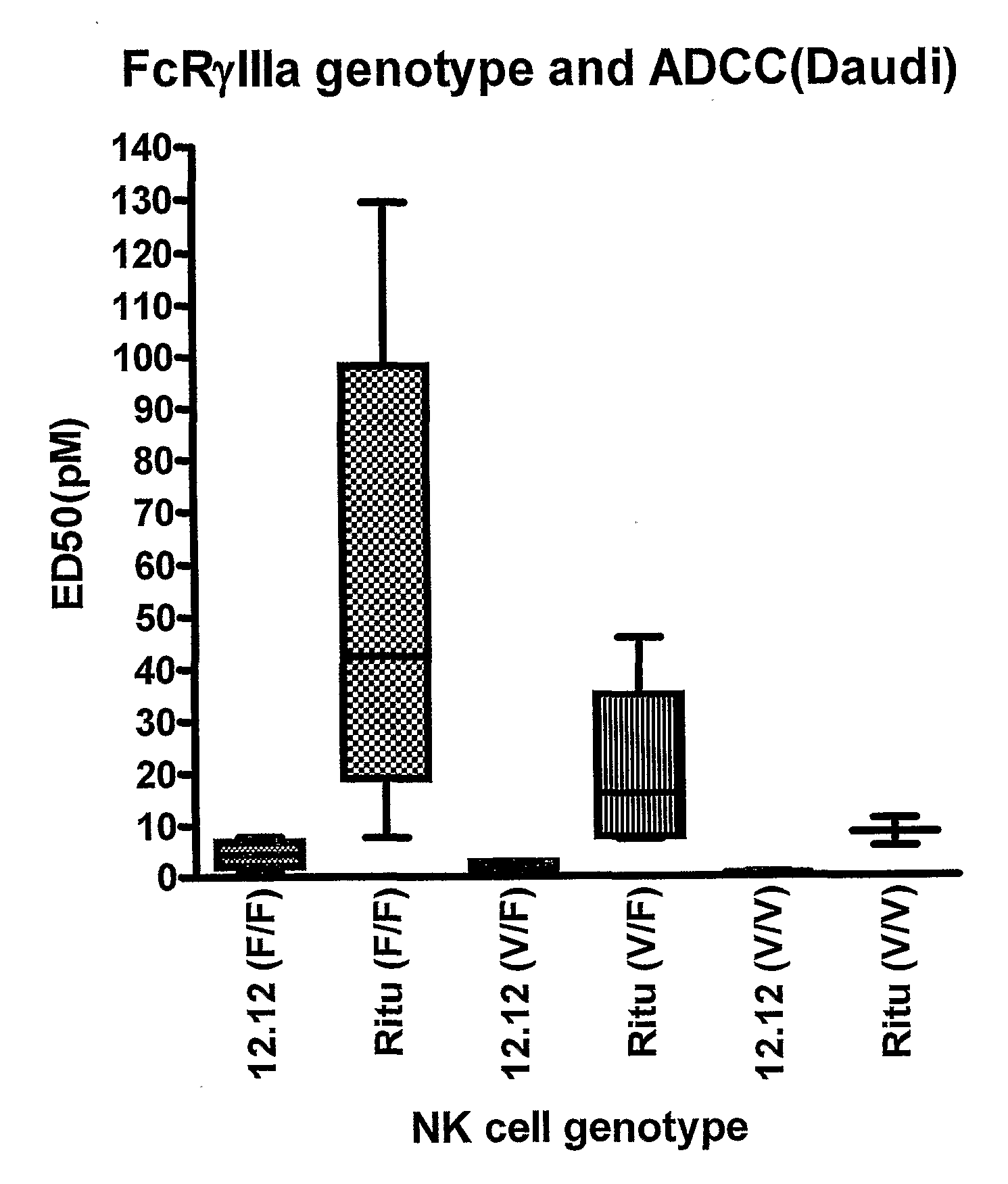
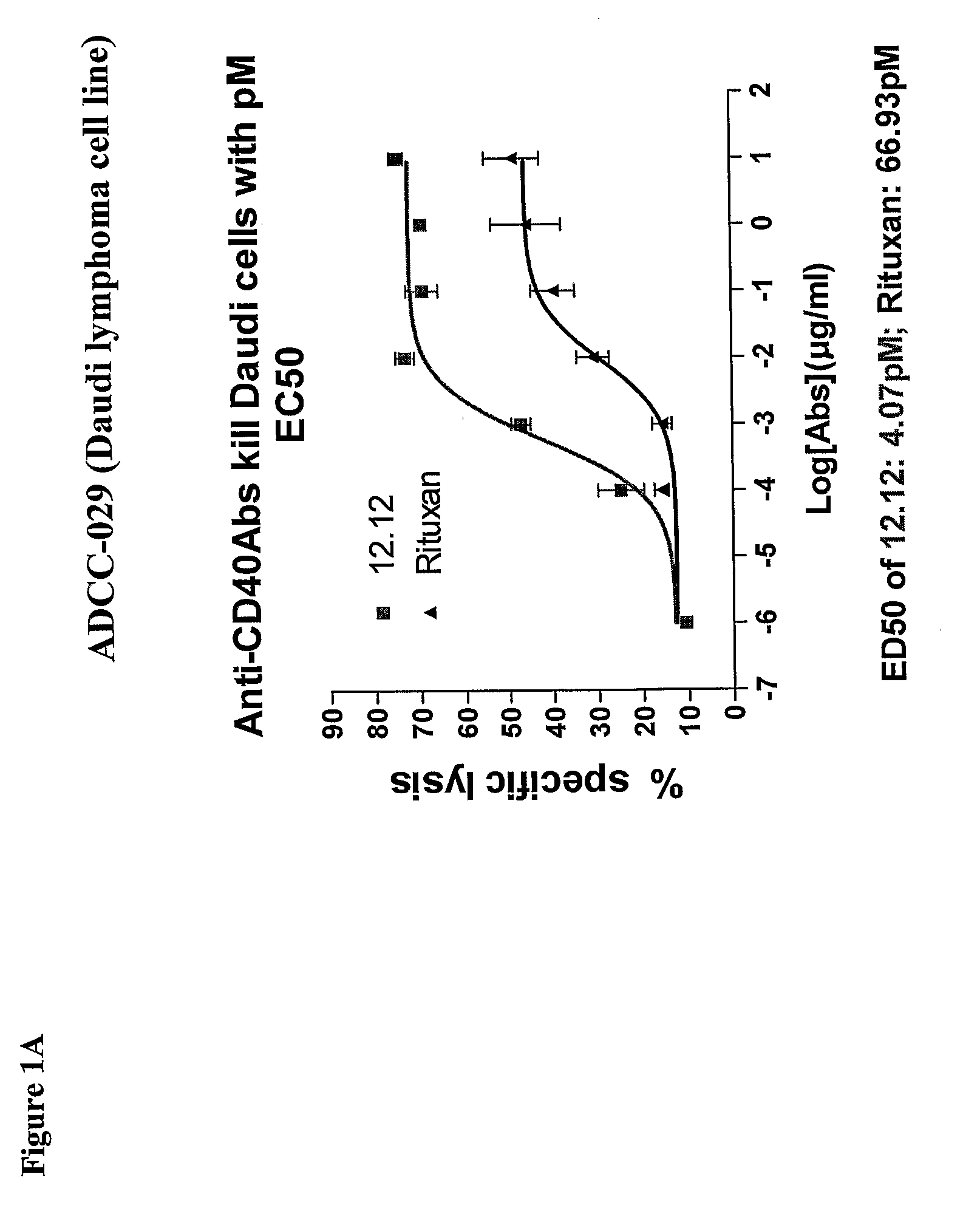
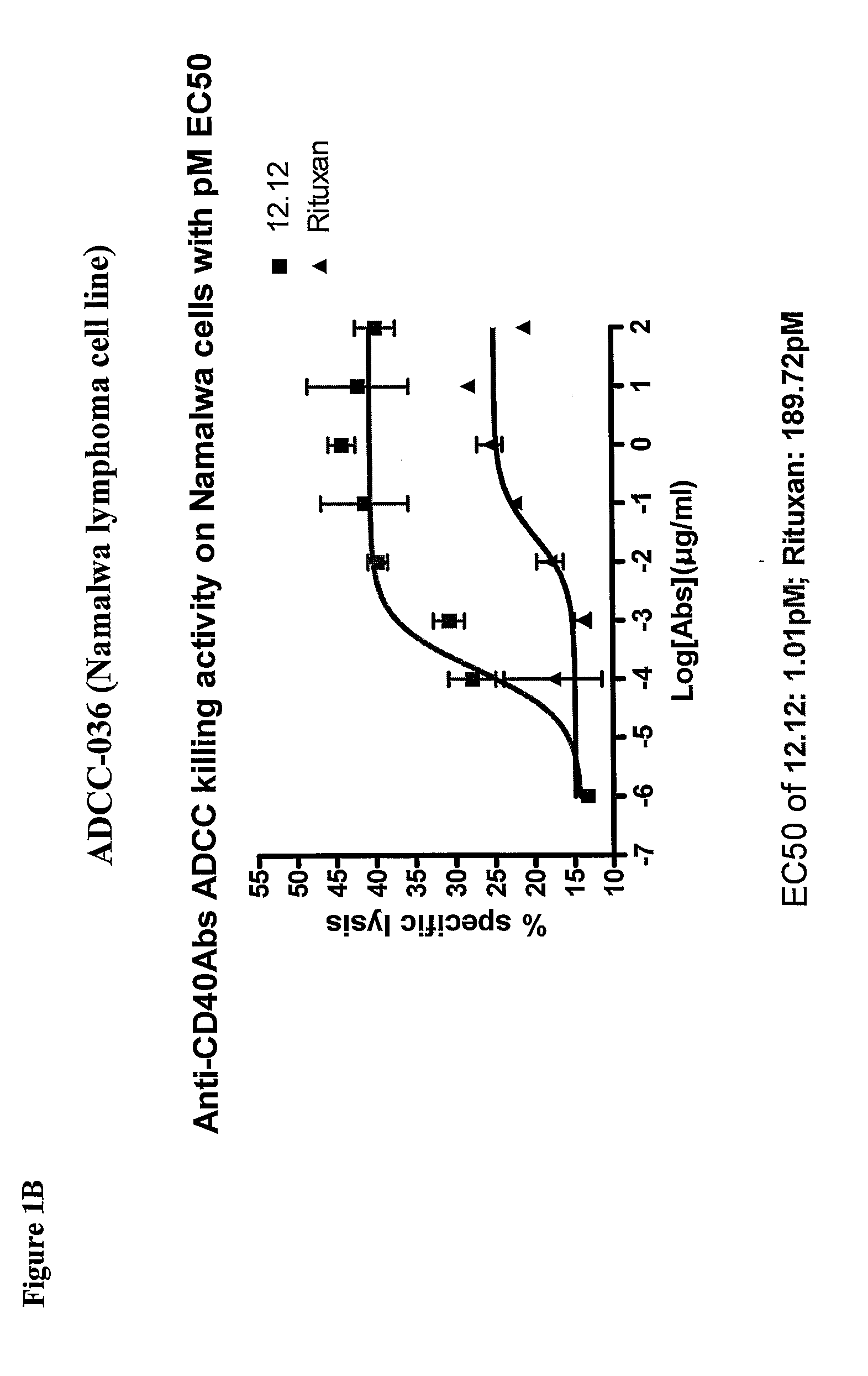
Methods for treating a human patient for a cancer or pre-malignant condition that is associated with CD40-expressing cells are provided, where the human patient is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). Also provided are methods of inhibiting antibody production by B cells in a human patient who is heterozygous or homozygous for FcγRIIIa-158F (genotype V / F or F / F). The methods comprise administering to the human patient a therapeutically or prophylactically effective amount of an anti-CD40 antibody. Methods and kits for identifying a human patient with a cancer or pre-malignant condition that is treatable with an anti-CD40 antibody and which is refractory to treatment with rituximab (Rituxan®), as well as methods and kits for selecting an antibody therapy for treatment of a human patient having a cancer or pre-malignant condition that is refractory to treatment with rituximab (Rituxan®), are also provided. The methods of the present invention find use in treatment of cancers and pre-malignant conditions that are associated with CD40-expressing cells. These methods are particularly advantageous with respect to cancers and pre-malignant conditions that are associated with cells expressing both CD40 and CD20, as the methods enable the treatment of patients having a cancer or pre-malignant condition that is refractory to therapy with other oncotherapeutic agents such as anti-CD20 antibodies.
Owner:XOMA TECH LTD
Method of promoting b-cell proliferation and activation with CD40 ligand and cyclosporin
InactiveUS6465251B1Efficient inductionModulate immune responseDead animal preservationArtificial cell constructsAntigenCyclosporins
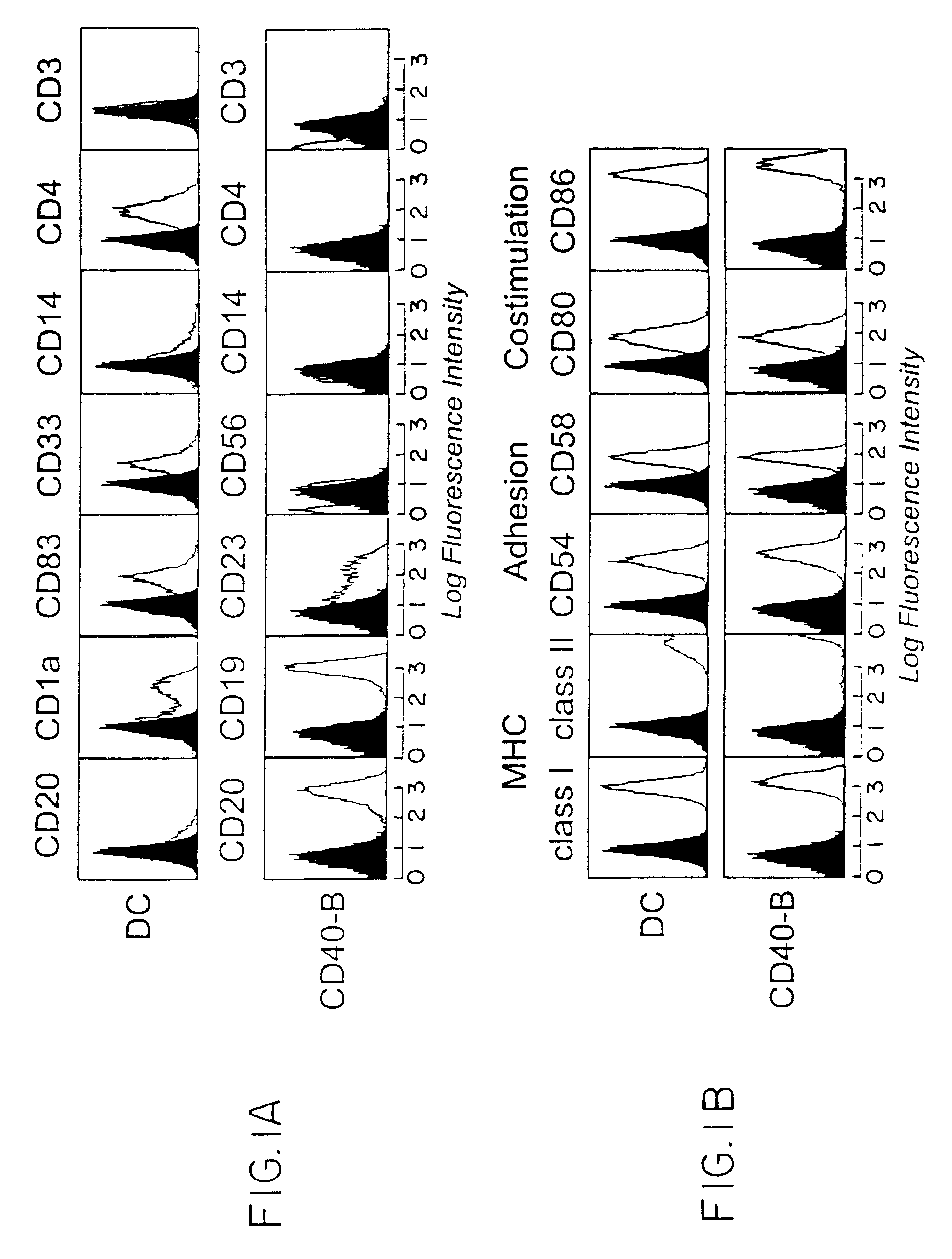
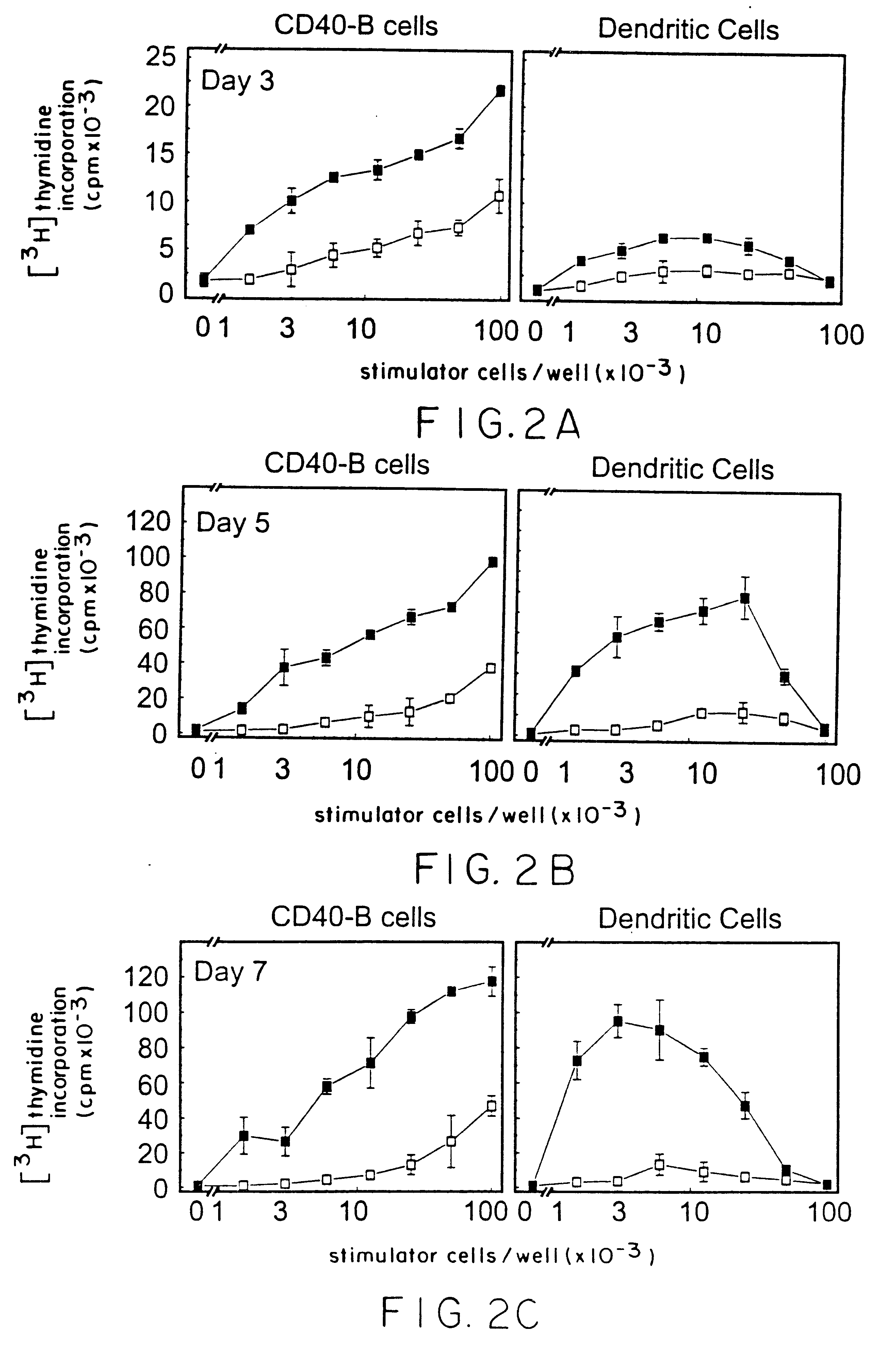
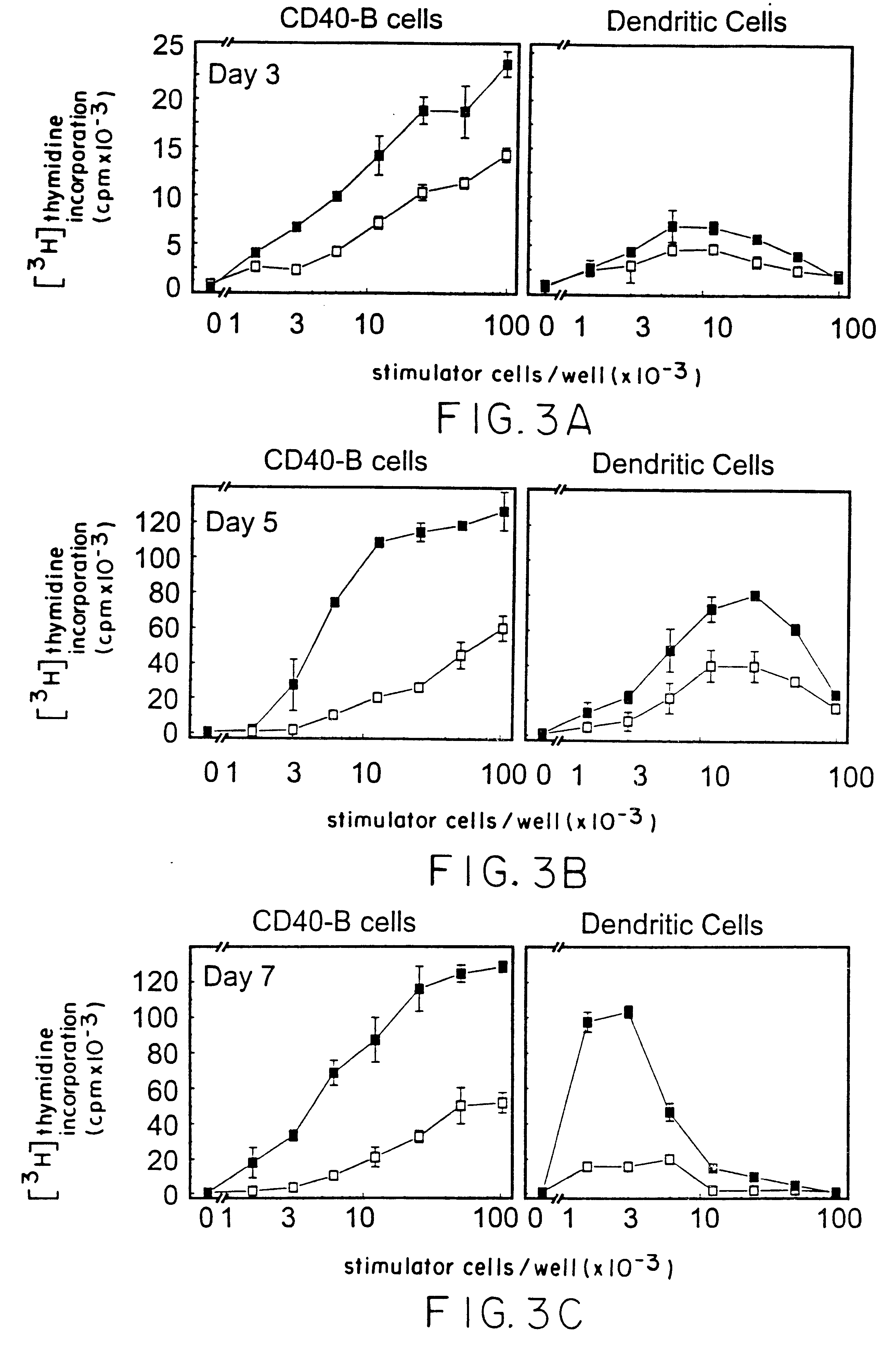
We teach a strategy to obtain large quantities of desired APCs, activated B cells, which are superior in their capacity to present tumor protein antigen in a multiadministration protocol. Human B cells can be obtained from peripheral blood in large numbers. These cells can be activated in vitro by coculture with CD40L (CD40-B cells) and an immunosuppressive agent such as cyclosporin A. They can expanded up to 1x103 to 1x104 fold in 2 weeks or 1x105 to 1x106 fold in 2 months. We demonstrate these cells are most efficient APCs comparable to DCs in stimulating allogeneic CD4+ CD45RA+, CD4+ CD45RO+, and CD8+ T cells. In contrast to DCs, CD40-B cells are fully functional even in the presence of immunosuppressive cytokines such as IL-10 and TGFbeta.
Owner:DANA FARBER CANCER INST INC
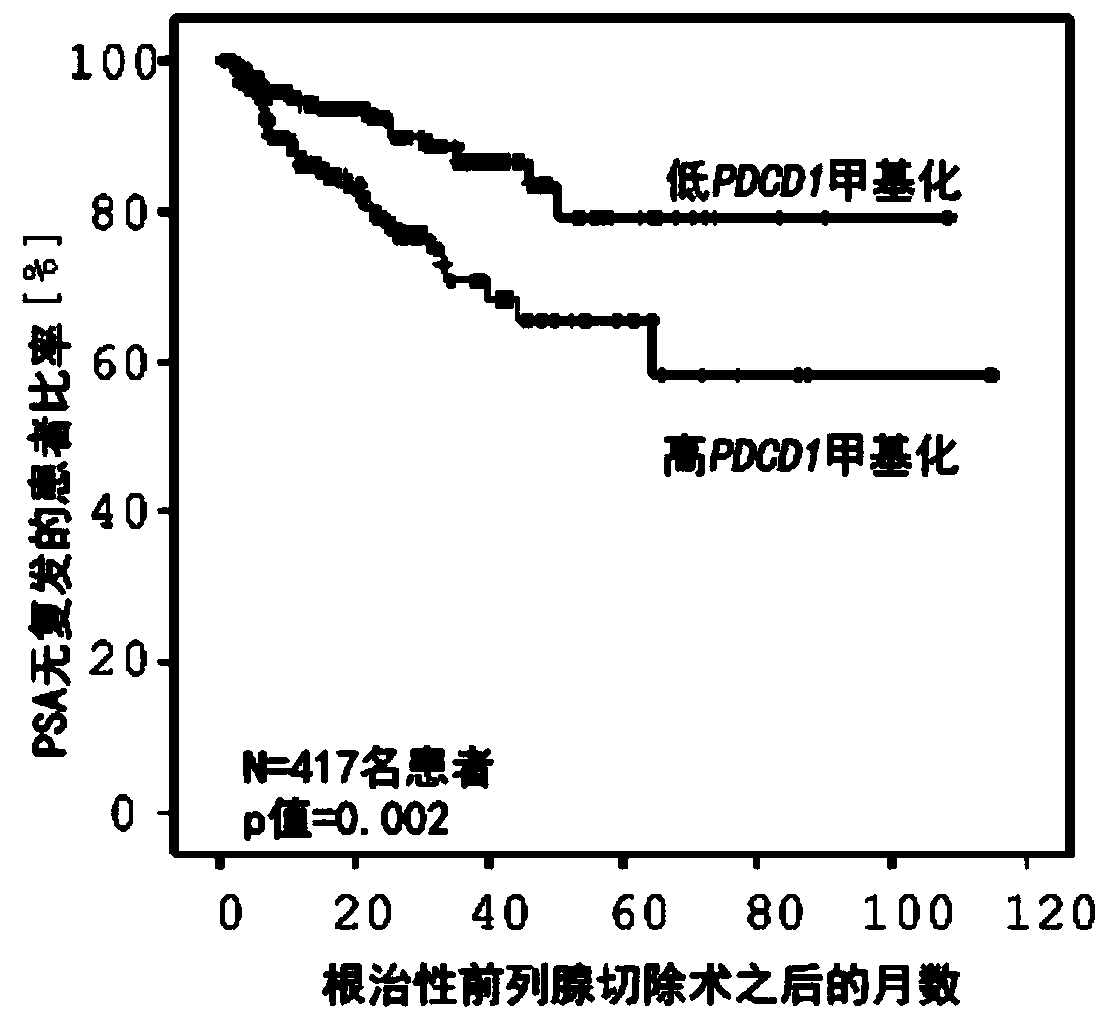
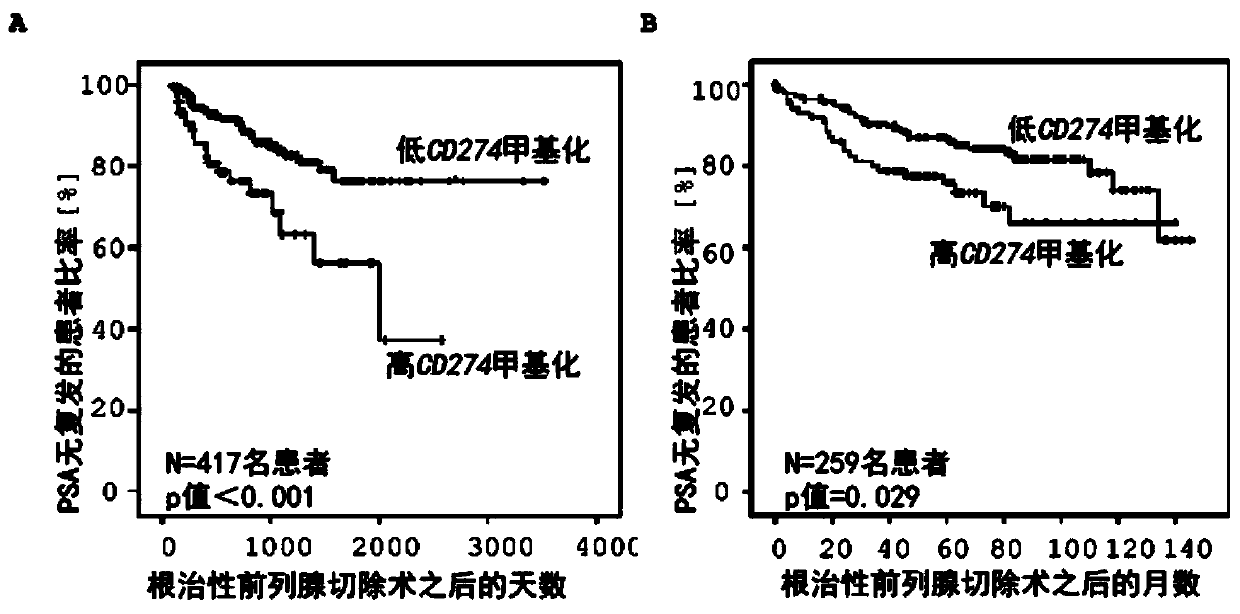
Method for assessing a prognosis and predicting the response of patients with malignant diseases to immunotherapy
The invention relates to, among others, a method for assessing a prognosis of a patient with a malignant disease and / or for predicting the response of a patient with a malignant disease to immunotherapy. For this purpose, a DNA methylation analysis is carried out on at least one immunoregulatory gene of cells of the malignant disease and / or T lymphocytes which interact with the cells of the malignant disease, said gene coding for an immune checkpoint selected from B7 proteins and the receptors thereof, MHC-peptide complex-binding co-receptors, the members of the tumor necrosis factor receptorsuperfamily TNFRSF9, CD40, TNFRSF4, TNFRSF18, and CD27, the members of the immunoglobulin superfamily TIGIT, BTLA, HAVCR2, BTNL2, and CD48, and the andenosine-binding adenosine 2A receptor.
Owner:迪莫迪特里希
Antibodies
ActiveUS20160311916A1Reduced bindingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibodyBiochemistry
Owner:ALLIGATOR BIOSCI
Antibodies to CD40
ActiveUS20070190051A1Organic active ingredientsFungiSingle-Chain AntibodiesIntravenous gammaglobulin
The present invention relates to antibodies and antigen-binding portions thereof that specifically bind to CD40, preferably human CD40, and that function as CD40 agonists. The invention also relates to human anti-CD40 antibodies and antigen-binding portions thereof. The invention also relates to antibodies that are chimeric, bispecific, derivatized, single chain antibodies or portions of fusion proteins. The invention also relates to isolated heavy and light chain immunoglobulins derived from human anti-CD40 antibodies and nucleic acid molecules encoding such immunoglobulins. The present invention also relates to methods of making human anti-CD40 antibodies, compositions comprising these antibodies and methods of using the antibodies and compositions for diagnosis and treatment. The invention also provides gene therapy methods using nucleic acid molecules encoding the heavy and / or light immunoglobulin molecules that comprise the human anti-CD40 antibodies. The invention also relates to transgenic animals comprising nucleic acid molecules of the present invention.
Owner:ABQENIX INC +1
Methods and Compositions for Producing an Enhanced Immune Response to a Human Papillomavirus Immunogen
InactiveUS20090068214A1Viral antigen ingredientsMicrobiological testing/measurementCo administrationHuman papillomavirus
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Methods and compositions for enhancing vaccine immune responses
ActiveUS20150202272A1Increase cytolytic activityIncrease immune responseSsRNA viruses negative-senseViral antigen ingredientsImmunogenicityT cell immunity
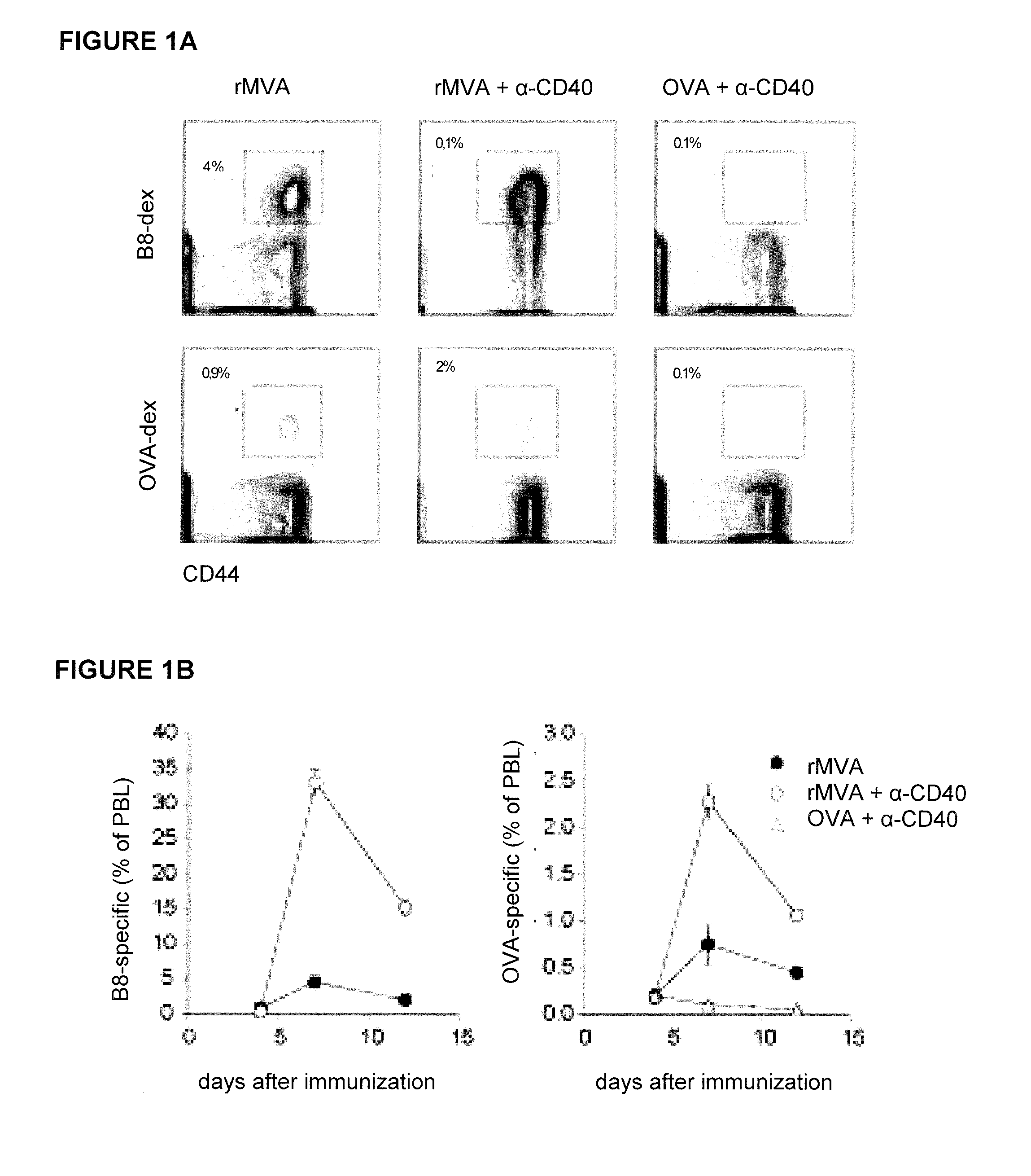
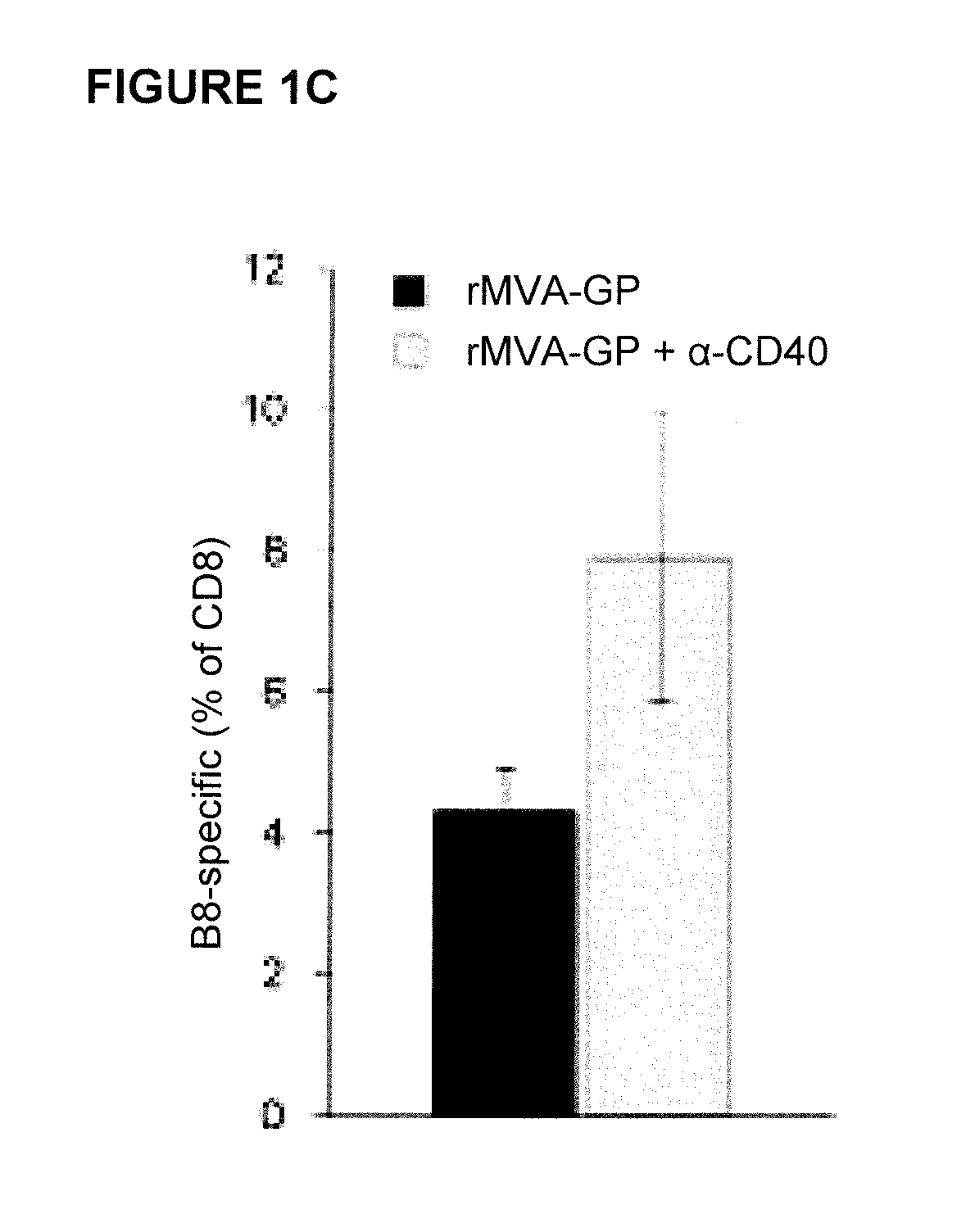
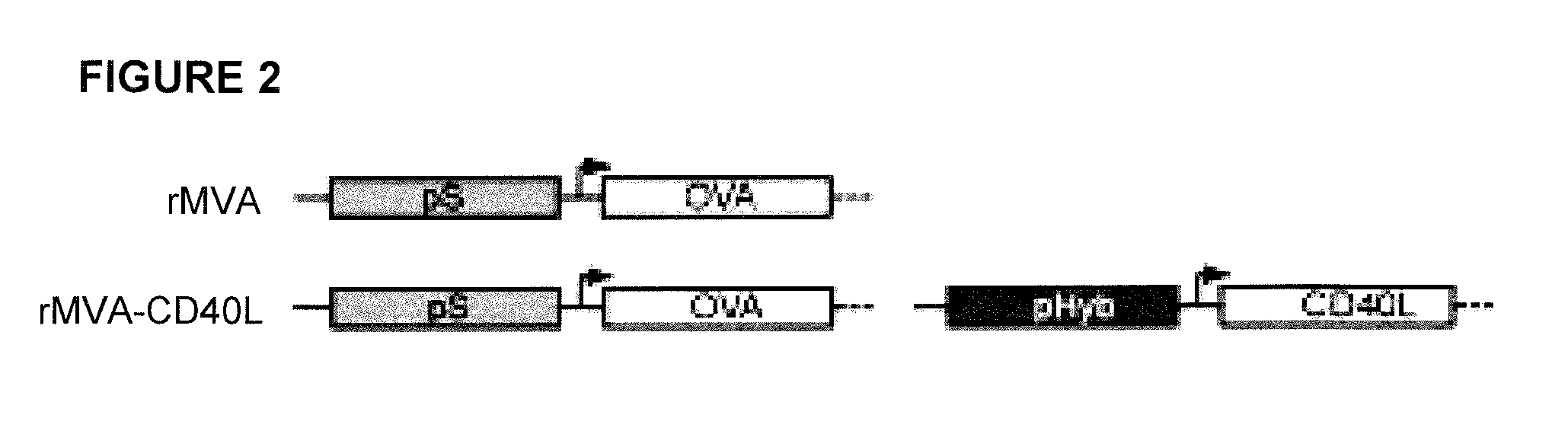
Provided herein are immunogenic compositions comprising a recombinant modified vaccinia virus Ankara (MVA) comprising a nucleic acid sequence encoding a CD40 ligand (CD40L) and a nucleic acid sequence encoding a heterologous disease-associated antigen, wherein the immunogenic composition induces increases T-cell immune responses specific for the heterologous disease-associated antigen when administered to a human host, and related methods and uses.
Owner:BAVARIAN NORDIC AS
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:DELUCIA DAVE
Membrane-anchored beta2 microglobulincovalently linked to MHC class I peptide epitopes
InactiveUS20080286312A1High level presentationAntibody mimetics/scaffoldsVirus peptidesMHC class ICell membrane
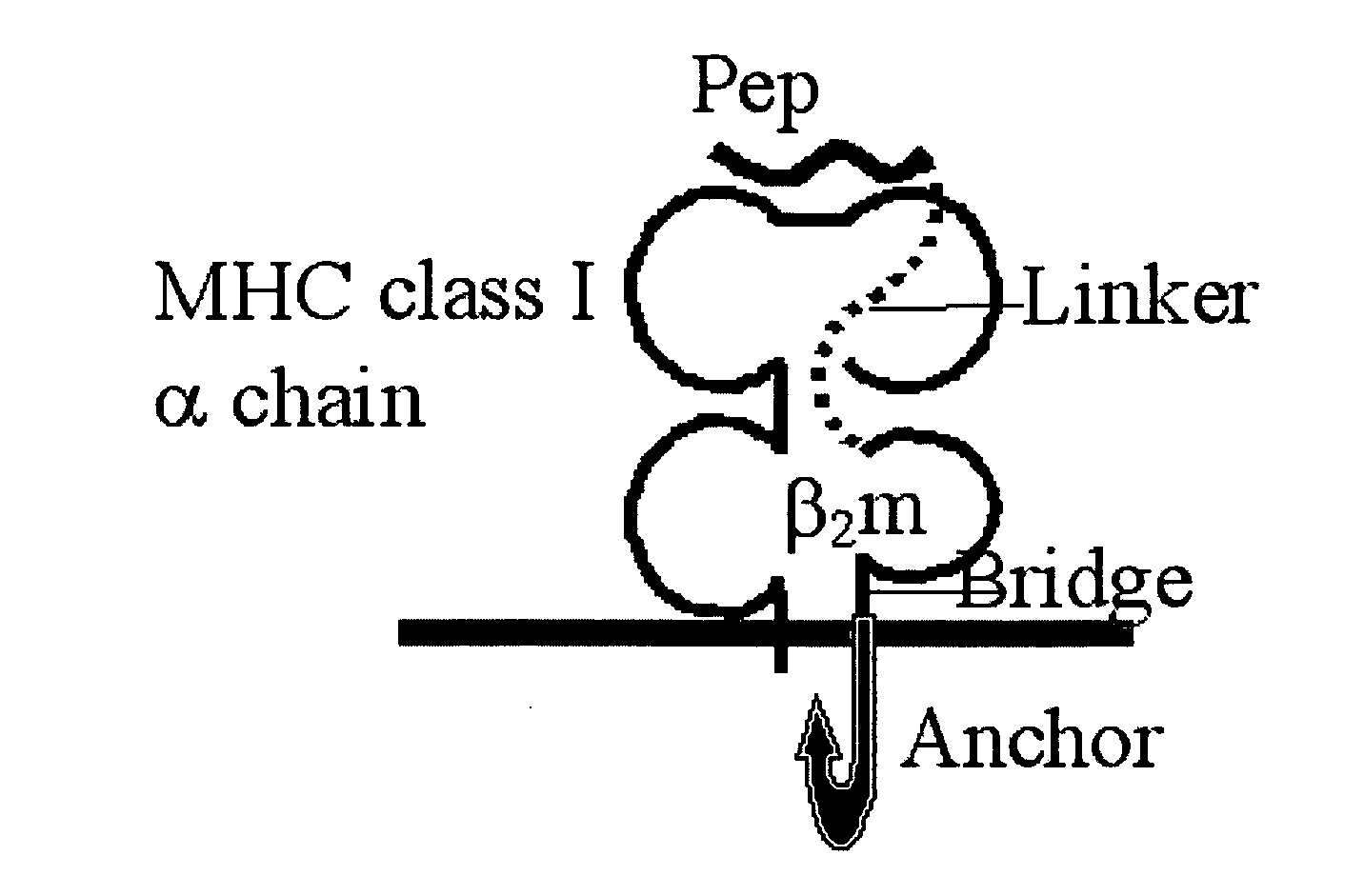
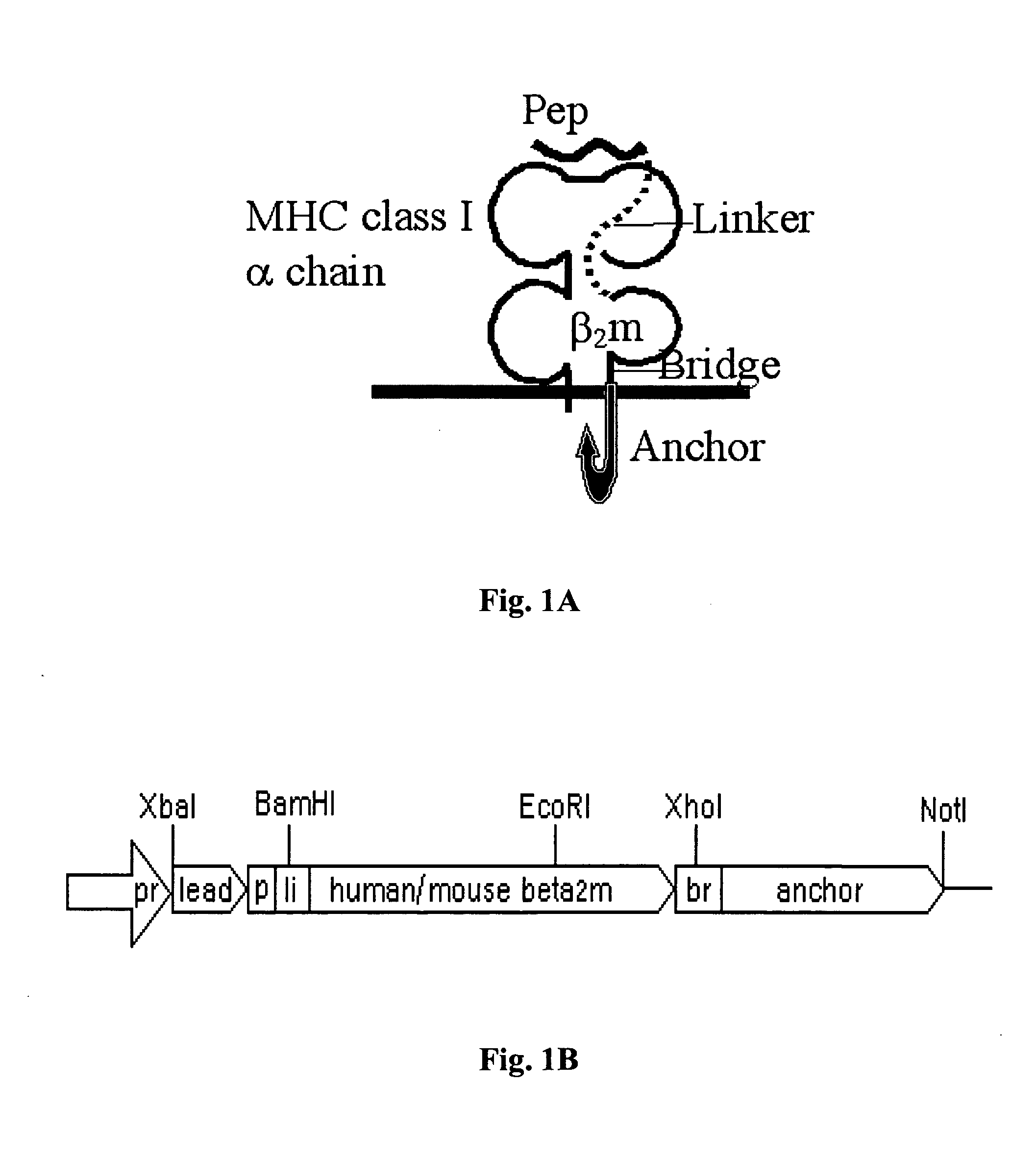
The invention provides a polynucleotide comprising a sequence encoding a polypeptide that is capable of high level presentation of antigenic peptides on antigen-presenting cells, wherein the polypeptide comprises a β2-microglobulin molecule that is linked through its carboxyl terminal to a bridge peptide which spans the whole distance to the cell membrane, said bridge peptide being linked to a polypeptide stretch consisting of the full or partial transmembrane and / or cytoplasmic domains selected from the group consisting of a toll-like receptor (TLR) polypeptide, a CD40 polypeptide, and TLR and CD40 polypeptides fused in tandem, that allows the anchorage of the β2-microglobulin molecule to the cell membrane, and through its amino terminal to at least one antigenic peptide comprising an MHC class I epitope, wherein said antigenic peptide is preferably derived from a tumor-associated antigen or from a pathogenic antigen. Antigen presenting cells and DNA and cellular vaccines for treatment of cancer and infectious diseases, are also provided.
Owner:GAVISH GALILEE BIO APPL
Agonistic antibodies that bind human cd40 and uses thereof
ActiveUS20180066053A1Enhance immune response against antigenIncreased activationNGF/TNF-superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibodyDiagnostic methods
Isolated monoclonal agonistic antibodies which bind to human CD40 and related antibody-based compositions and molecules are disclosed. Also disclosed are therapeutic and diagnostic methods for using the antibodies.
Owner:CELLDEX THERAPEUTICS INC
Compositions and methods for treating cancer
Use of a CXCR4 antagonistic peptide and an immune-check point regulator in the treatment of cancer is provided. Accordingly there is provided a method of treating cancer in a subject in need thereof, the method comprising administering to the subject a therapeutically effective amount of a peptide having an amino acid sequence as set forth in SEQ ID NO: 1 or an analog or derivative thereof; and a therapeutically effective amount of a PD1 antagonist, a PDL-1 antagonist, a CTLA-4 antagonist, a LAG-3 antagonist, a TIM-3 antagonist, a KIR antagonist, an IDO antagonist, an OX40 agonist, a CD137 agonist, a CD27 agonist, a CD40 agonist, a GITR agonist, a CD28 agonist or an ICOS agonist, thereby treating the cancer in the subject. Also provided are pharmaceutical compositions and articles of manufacture.
Owner:BIOKINE THERAPEUTICS LTD +1
Mucin antigen vaccine
Provided are expression vectors for generating an immune response to a mucin. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal, a mucin antigen and CD40 ligand. Also provided are methods of generating an immune response against cells expressing a mucin by administering an effective amount of the vector. Further provided are methods of generating an immune response against cancer cells expressing a mucin in an individual by administering an effective amount of the vector. Still further provided are methods of overcoming anergy to a mucin self antigen by administering an effective amount of the vector.
Owner:MICROVAX LLC
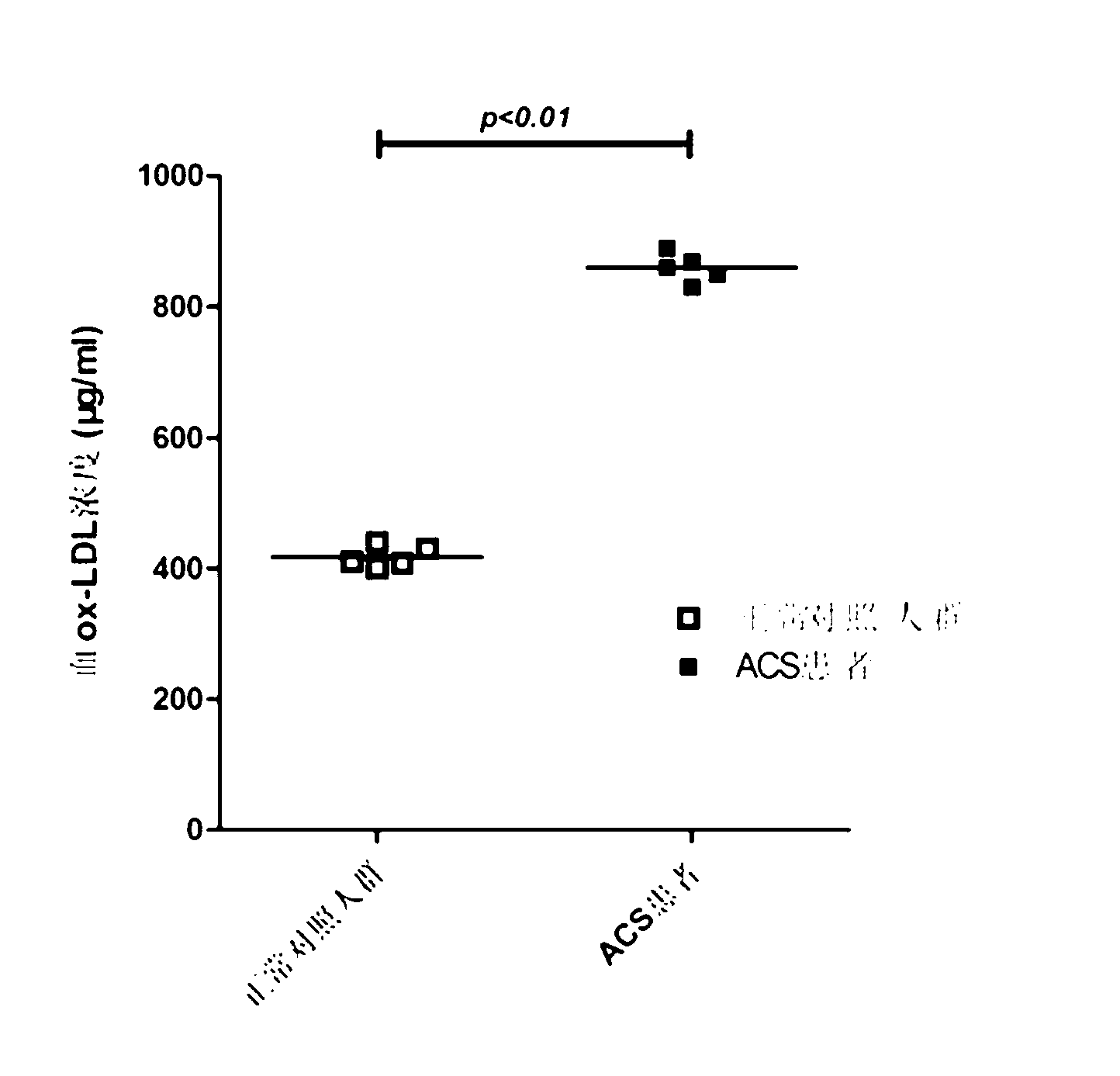
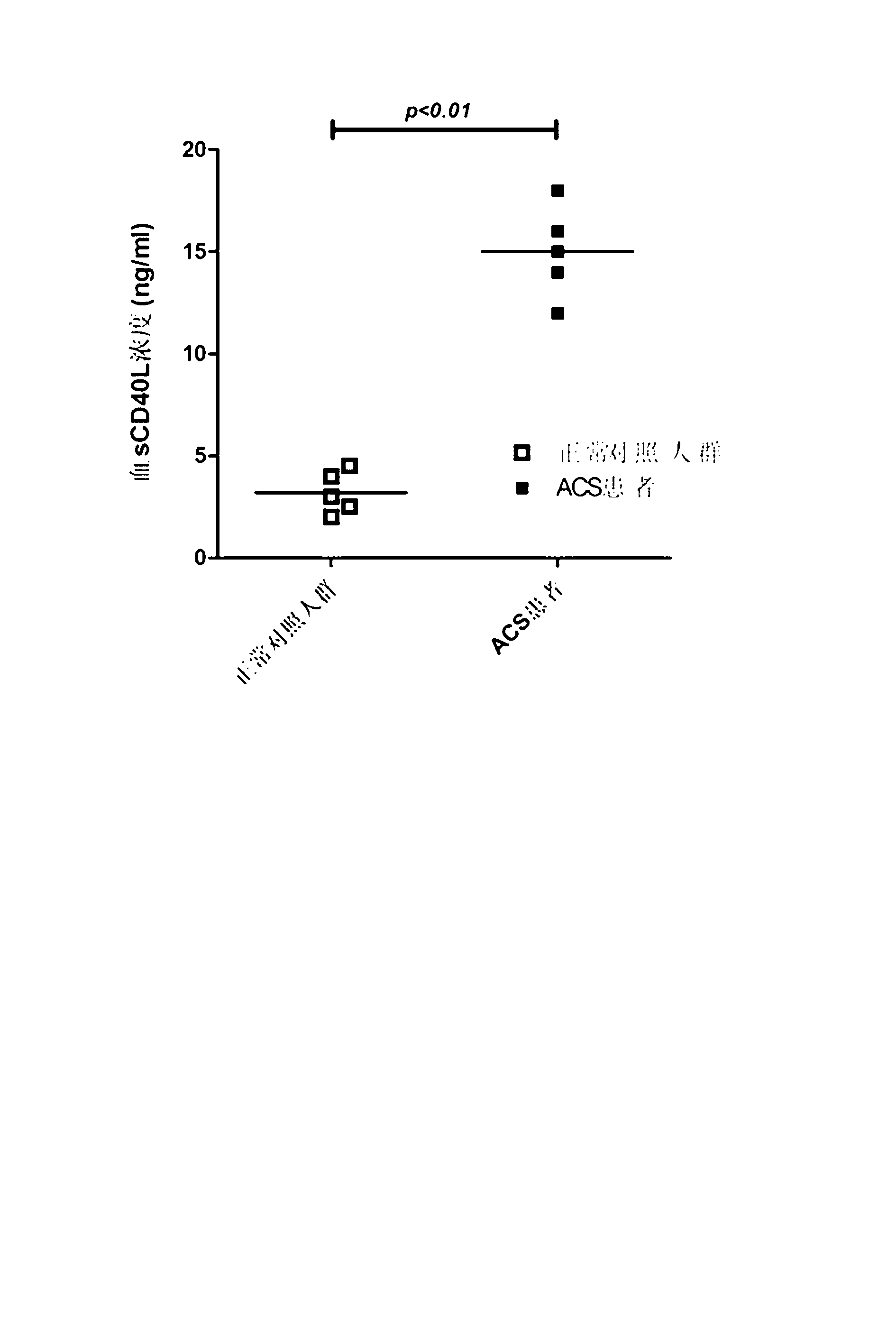
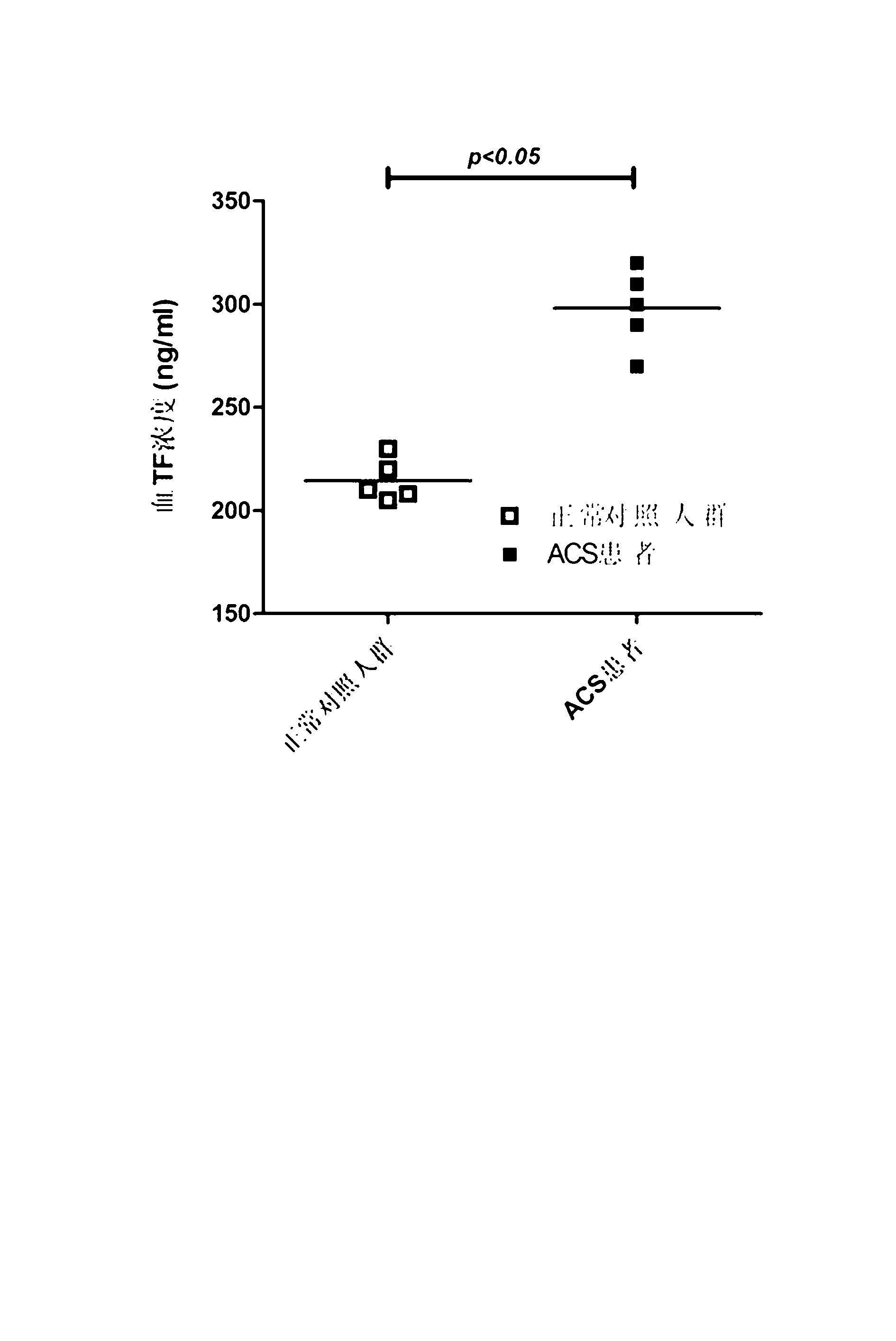
Liquid-phase chip kit for acute coronary syndrome and preparation method for same
InactiveCN103163295AComprehensive and comprehensive assessment of prognosisImprove detection efficiencyMaterial analysisA lipoproteinMicrosphere
The invention relates to a liquid-phase chip kit for acute coronary syndrome and a preparation method for the same. A liquid-phase chip mainly comprises microspheres coated with oxidized low density lipoprotein (ox-LDL), soluble CD40 ligand (sCD40L), matrix metallopeptidase 9 (MMP-9), tissue factor (TF), omentin and endogenous secretory receptor of advanced glycation end products (esRAGE) capture antibody respectively, detection antibodies labelled by biotin, and streptavidin phycoerythrin. The liquid-phase chip provided by the invention has the advantages of being high in detection efficiency, low in the quantity of the needed samples, strong in specificity, high in sensitivity, rapid and accurate in detection, and the like. Simultaneously, the chip reflects a protective factor level and an injury factor level in an organism, so that the risk stratification and prognosis conditions of a patient can be much comprehensively assessed. Simultaneously, the preparation method disclosed by the invention is simple and practicable, and good in stability, various process parameters such as the quantities of the microspheres and the antibodies, and the reaction process in the technical scheme of the preparation method are obtained on the basis of lots of experiments, and are the optimal parameter values of the preparation process.
Owner:吴宗贵 +2
Immunomodulation by genetic modification of dendritic cells and B cells
InactiveUS6841540B1Improving gene transferEnhanced allo-MLRBiocideAntibody mimetics/scaffoldsGene deliveryDendritic cell
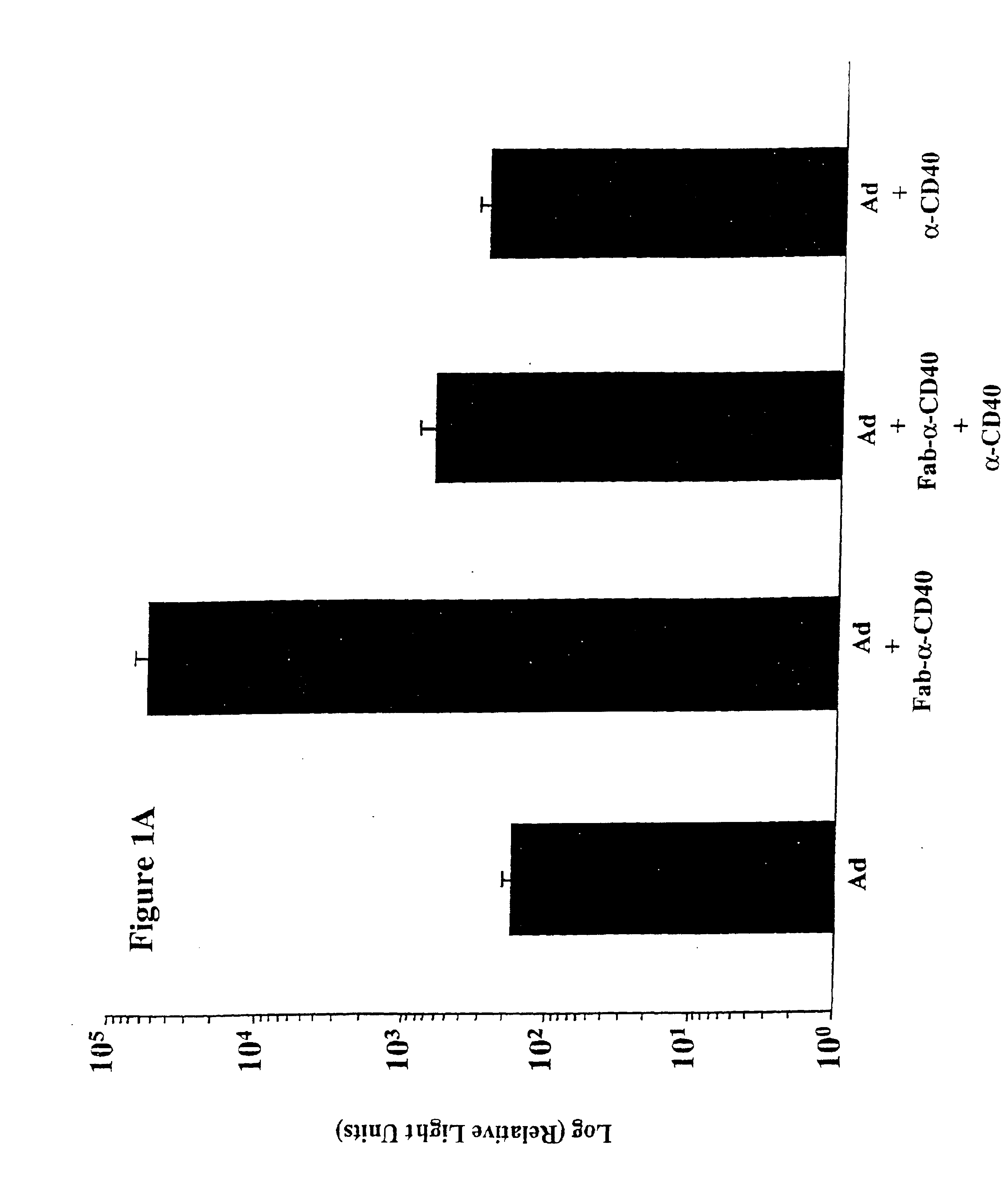
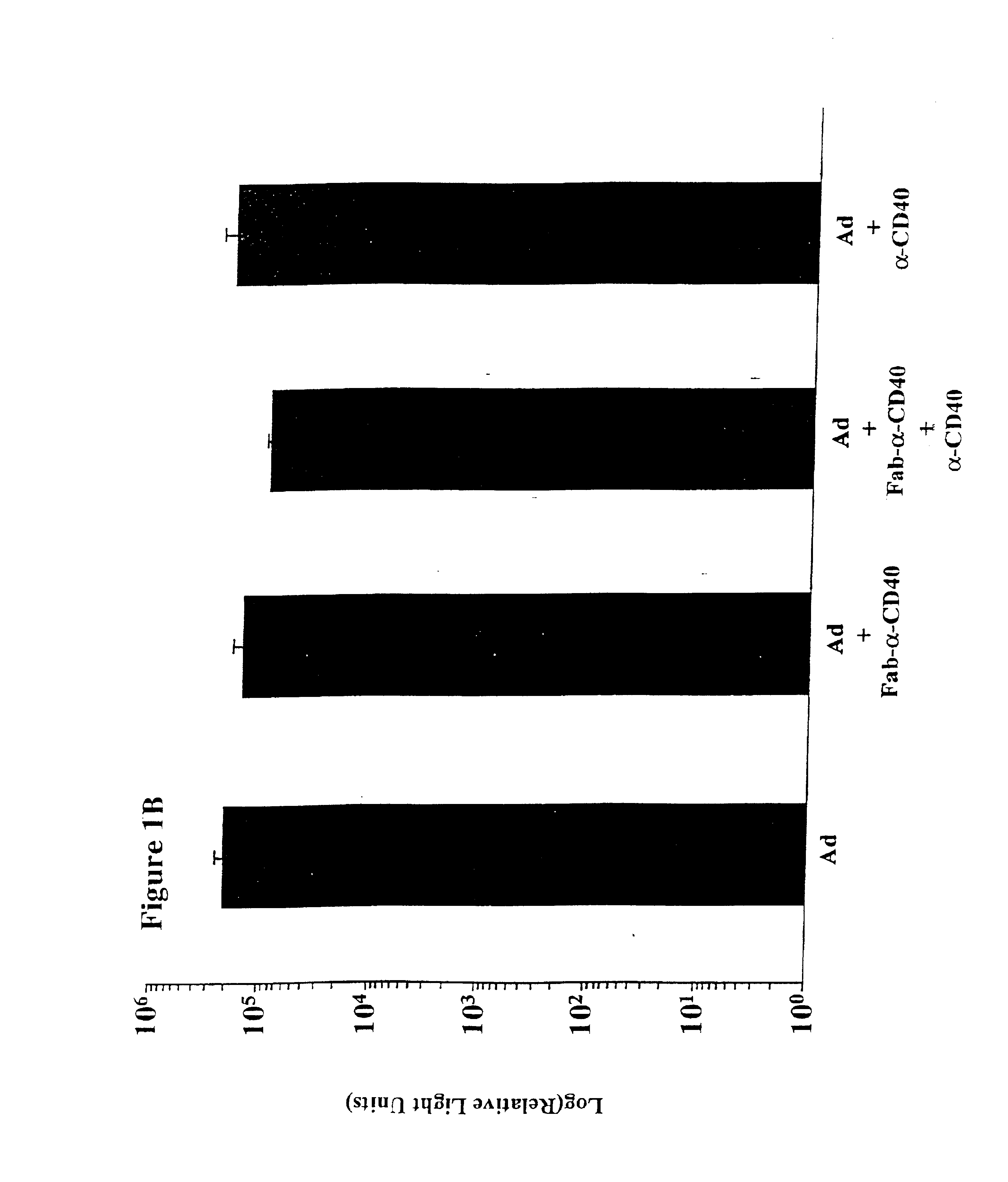
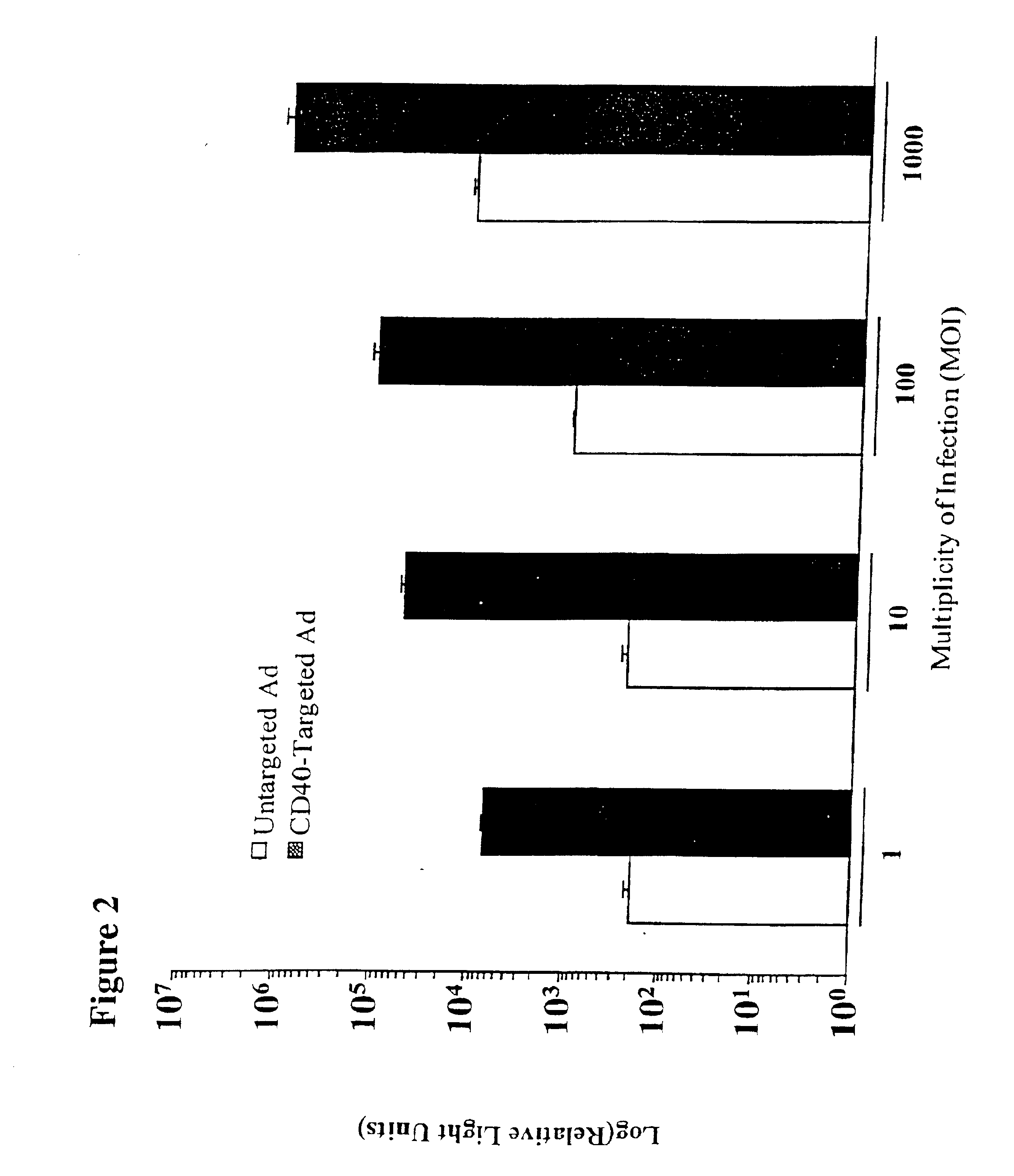
The present invention provides a CD40-targeted gene delivery system and a CD40-targeted recombinant adenoviral vector for genetic manipulation of dendritic cells and B cells. Also provided are methods of using this enhanced gene delivery to immune system cells and therefore, enhancing dendritic cell-based immunotherapy.
Owner:UAB RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com