Method for assessing a prognosis and predicting the response of patients with malignant diseases to immunotherapy
A technology for immunotherapy and disease, applied in the field of implementing the method or the kit for the use, and molecular diagnosis, which can solve the problems that clinicians do not have, lack of prognosis and prediction methods, and inaccurately estimate the course of disease of individual patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
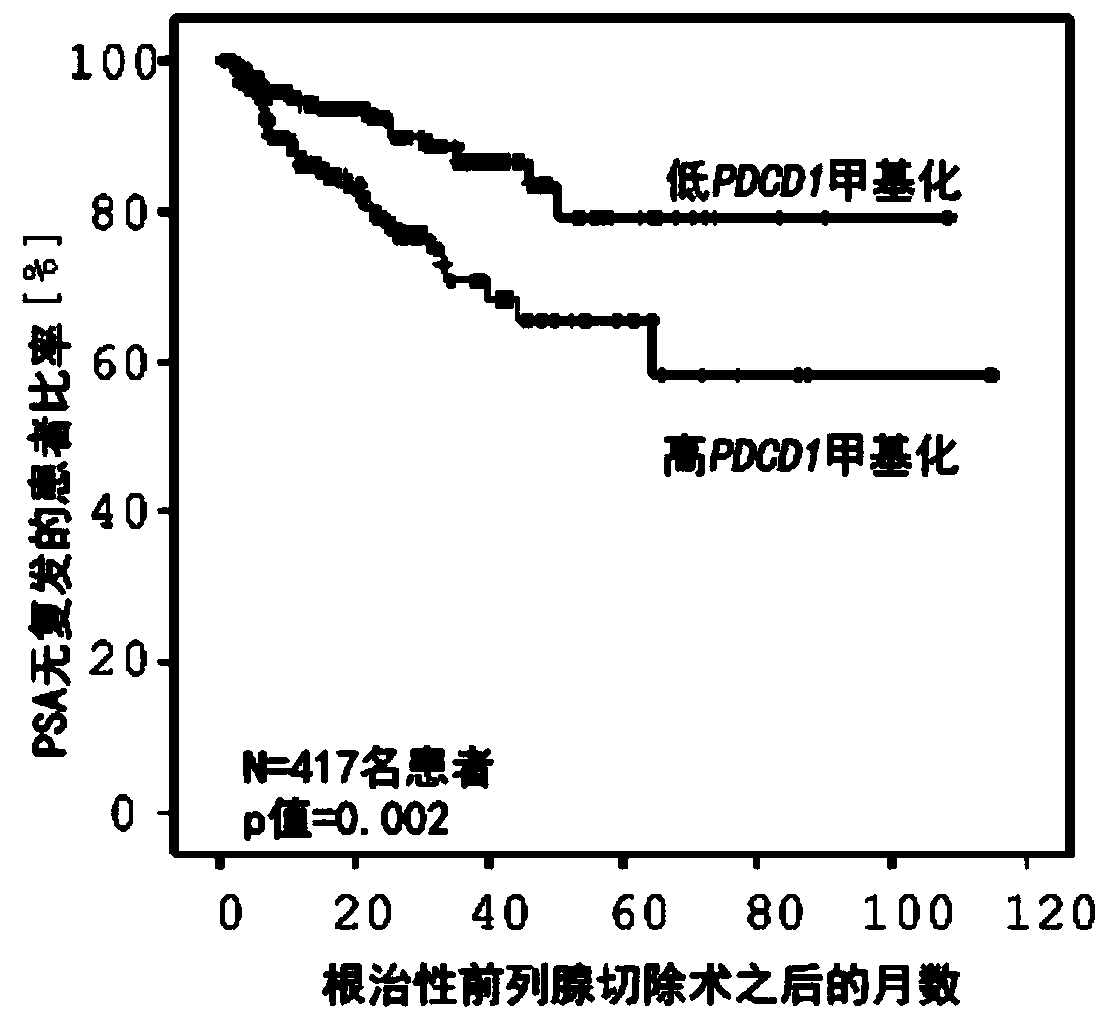
[0135] Example 1: Determining the prognosis of patients after radical resection of prostate cancer based on the DNA methylation analysis of the immune regulatory gene PDCD1
[0136] According to a method embodiment of the present invention, determination of the prognosis of a patient may be performed in locally confined (localized, non-spread) prostate cancer, for example after radical prostatectomy and radical resection of tumors located in the prostate rear.
[0137] First, DNA is obtained from the tumor. For example, proteinase K can be used to lyse tumor tissue, followed by silica spin column extraction of DNA. For obtaining DNA from tumors, commercially available kits such as QIAamp DNA Mini Kit (Qiagen N.V., Helden, Germany) are commercially available. Subsequent bisulfite conversion of the extracted DNA deaminates essentially all cytosines to uracil, leaving methylated cytosines unchanged. Methylation analysis is then performed using, for example, the Infinium HumanM...
Embodiment 2
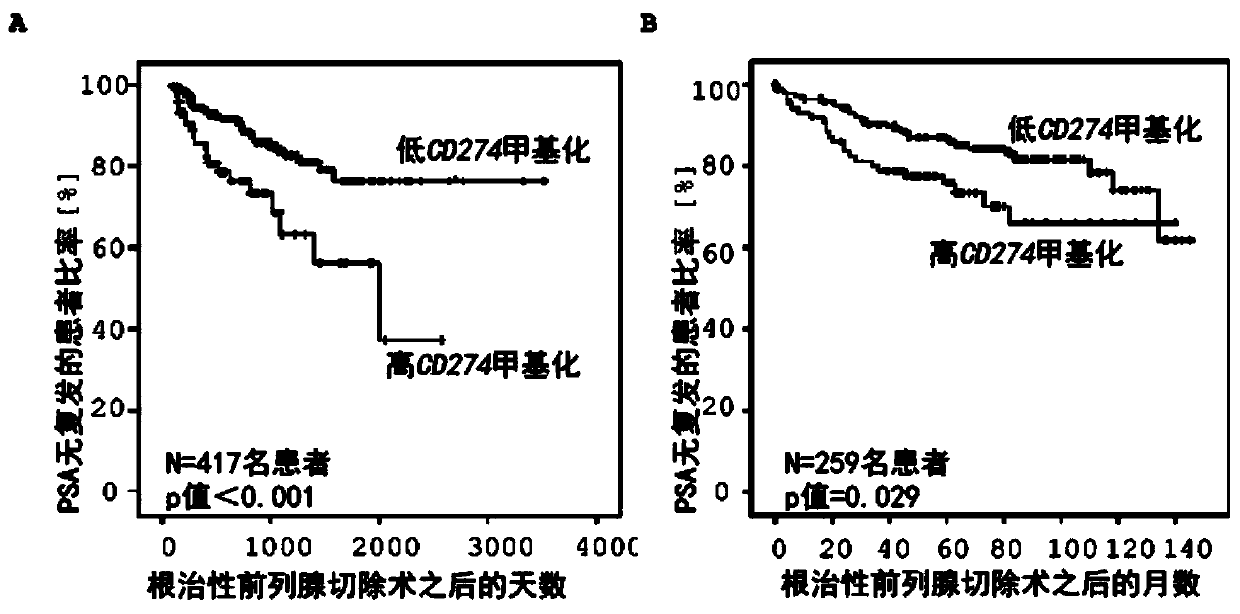
[0141] Example 2: Determining the prognosis of prostate cancer patients after radical resection based on DNA methylation analysis of the immune regulatory gene CD274
[0142] By implementing the method according to the present invention, it is also possible to determine the prognosis of patients with malignant diseases through DNA methylation analysis of CpG dinucleotides within the CD274 gene. For example, the prognosis of patients with locally restricted prostate cancer was obtained based on the methylation of the CD274 gene within the tumor after the patient underwent complete removal (radical resection) of the prostate and tumors located in the prostate.
[0143] In total, tumor samples from 417 patients with known prognosis were analyzed retrospectively. DNA methylation analysis was performed as described in Example 1. In order to calculate the relative methylation value of the CD274 gene locus based on the Infinium HumanMethylation450BeadChip raw data, five bead pairs c...
Embodiment 3
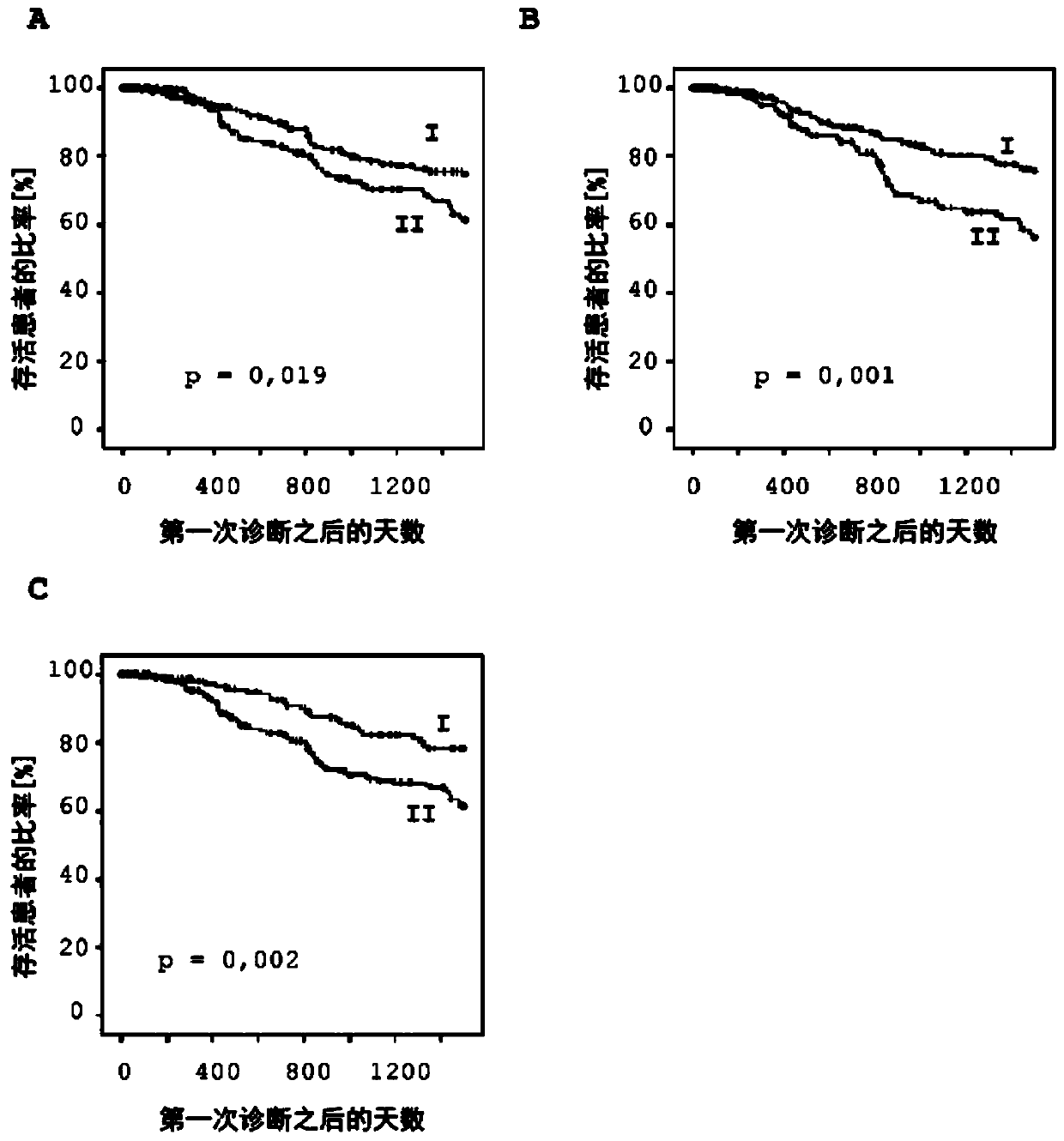
[0150] Example 3: Determining the prognosis of malignant melanoma patients based on DNA methylation analysis of the immune regulatory genes PDCD1, PDCD1LG2 and CD274
[0151] This example shows the determination of the prognosis of 470 patients with malignant melanoma according to the present invention. The results of the DNA methylation analysis were generated using the Infinium HumanMethylation 450 BeadChip as described in Example 1. To determine the relative methylation values of the corresponding genes, the relative methylation values were first determined as described in Example 1 for each selected bead of the Infinium HumanMethylation450BeadChip. The relative methylation values of up to six bead pairs per gene were then averaged to obtain representative methylation values for a specific gene. In order to carry out methylation analysis to CD274, use bead pair cg15837913 (SEQ ID NO:8), cg02823866 (SEQ ID NO:9), cg14305799 (SEQ ID NO:10), cg13474877 (SEQ ID NO:11) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com