Biological amnion and preparation method thereof
A kind of amniotic membrane and biological technology, applied in the direction of medical science, surgery, layered products, etc., can solve the problems of unimproved single-layer amniotic membrane, the natural structure of amniotic membrane is greatly damaged, and the arrangement of collagen bundles is irregular, so as to increase product thickness and reliability. Operability, good decellularization effect, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1. Raw material pretreatment: remove surface blood stains from fresh amnion, and wash with phosphate buffer;
[0034] Step 2. Virus inactivation: soak the amniotic membrane in step 1 in 75% ethanol solution at room temperature 25°C for 2 hours, and wash with purified water 4 times;
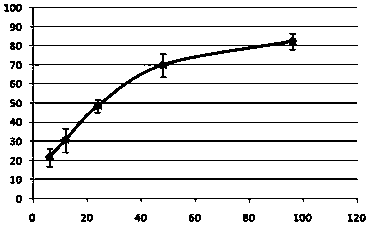
[0035] Step 3, decellularization treatment: place the amniotic membrane in step 2 in a hypertonic solution (the hypertonic solution is a mixed solution of 4M NaCl and 0.05M NaOH) at a room temperature of 25°C, shake at room temperature for 2.5 hours, and then quickly wash with purified water. Then use purified water to shake at room temperature for 2.5 hours; repeat once to prepare the amnion layer;
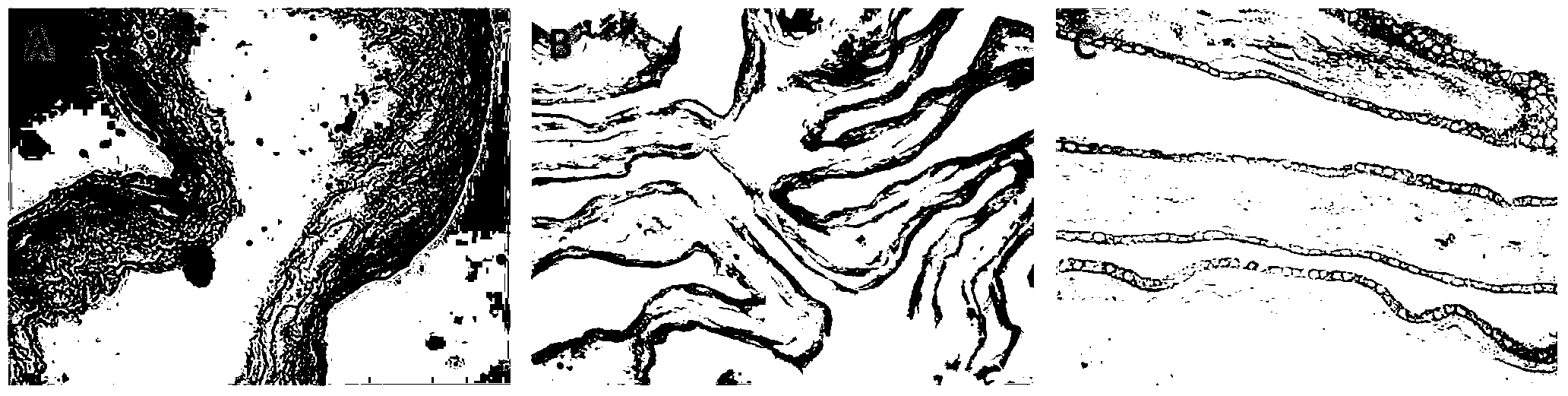
[0036] Step 4. Collagen layer preparation: wash the amnion layer prepared in step 3 and place the epithelial surface (smooth side) down on the bottom of a container with the same size as the amnion, add collagen solution, the thickness is about 10.0 μm, -20 Pre-freeze at ℃ for 2 hours an...
Embodiment 2
[0041] Step 1. Raw material pretreatment: remove surface blood stains from fresh amniotic membrane and wash with purified water;
[0042] Step 2. Virus inactivation: soak the amniotic membrane in step 1 in 0.5% peracetic acid solution for 10 minutes at room temperature 25°C, and wash with purified water 4 times;
[0043] Step 3, decellularization treatment: place the amniotic membrane in step 2 at room temperature 25°C in a hypertonic solution (the hypertonic solution is a mixed solution of 1M NaCl and 0.10M NaOH), shake at room temperature for 0.5h, wash it with purified water quickly, and then Use purified water to shake at room temperature for 0.5 h; repeat 5 times to prepare the amnion layer;
[0044] Step 4. Preparation of collagen layer: Wash the amnion layer prepared in step 3 and place the epithelial surface (smooth side) downward on the bottom of a container with the same size as the amnion, add collagen solution, the thickness is about 40.0 μm, -20 After pre-freezin...
Embodiment 3
[0049] Step 1. Raw material pretreatment: remove surface blood stains from fresh amniotic membrane and wash with normal saline;
[0050] Step 2. Virus inactivation: soak the amniotic membrane in step 1 in 75% ethanol solution for 3 hours at room temperature of 25°C, and wash with purified water for 5 times;
[0051] Step 3, decellularization treatment: place the amniotic membrane in step 2 in a hypertonic solution (hypertonic solution is a mixed solution of 3M NaCl and 0.01M NaOH) at room temperature at 25°C, shake at room temperature for 1 hour, wash with purified water quickly, and then use Shake the purified water at room temperature for 1 hour; repeat 4 times to prepare the amnion layer;
[0052] Step 4. Preparation of collagen layer: Wash the amnion layer prepared in step 3 and place the epithelial surface (smooth side) downward on the bottom of a container with the same size as the amnion, add collagen solution, the thickness is about 30.0 μm, -20 After pre-freezing at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com