Drug-carrying liposome modified by antimicrobial peptide and preparation method and application thereof
A technology of antimicrobial peptides and liposomes, applied in the field of biomedicine, can solve the problems of limited application, toxicity and death, hepatic hemorrhage, etc., and achieve the effects of alleviating drug resistance, high antibacterial activity, and reducing the number of bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
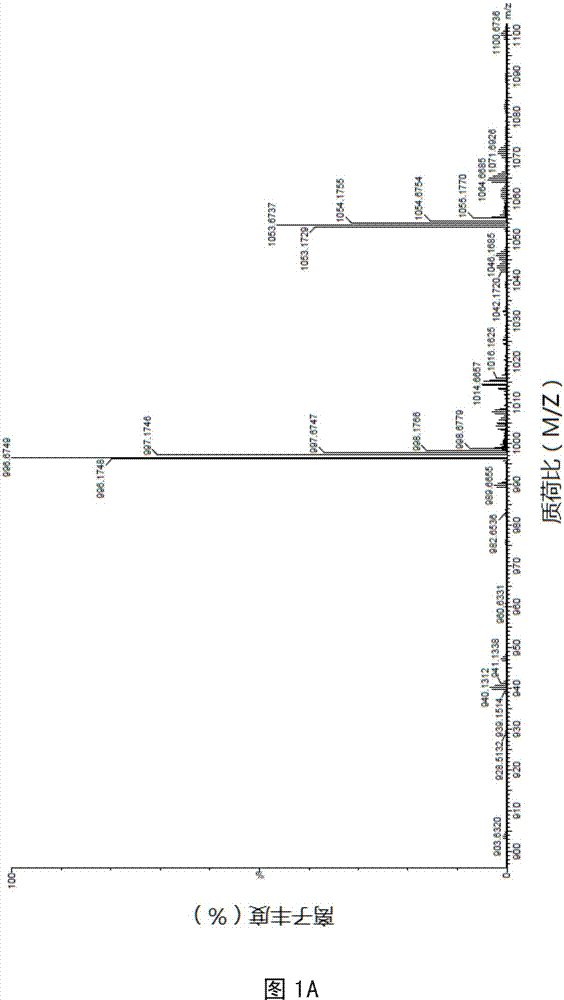
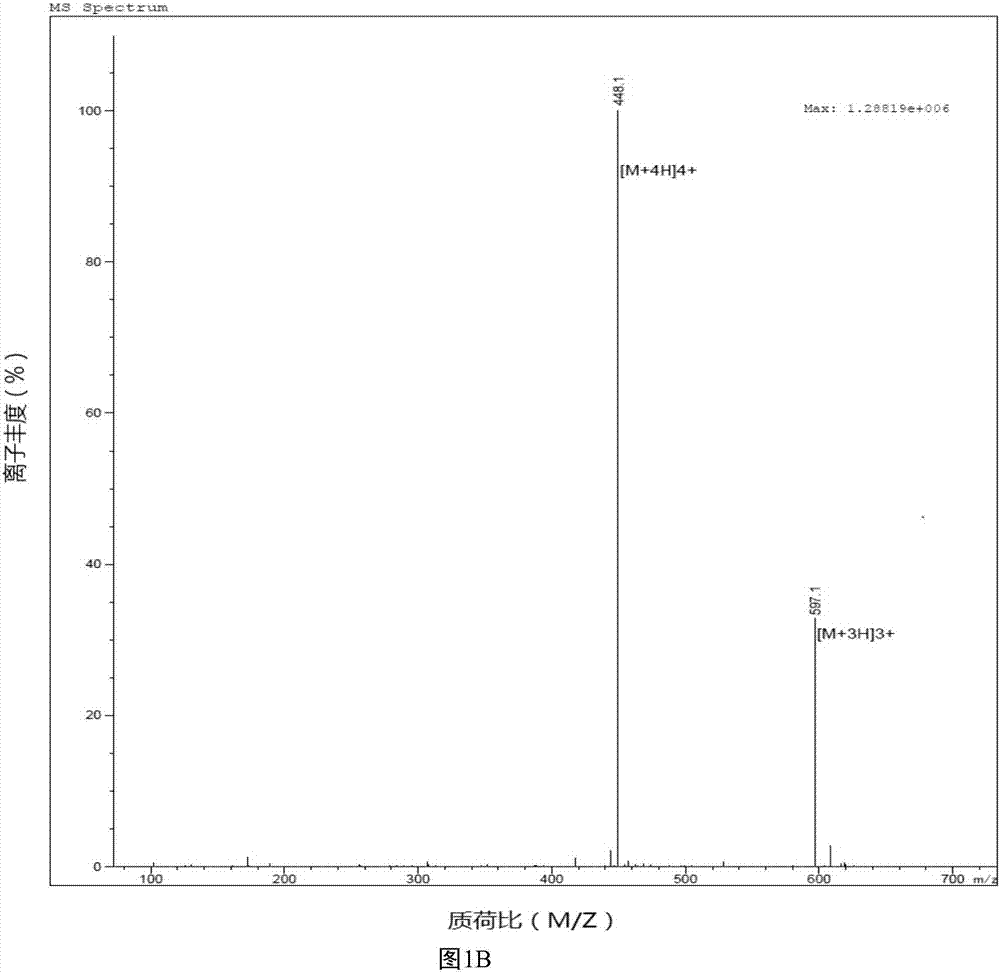
[0064] Example 1 Synthesis of Antimicrobial Peptide DP7 and Cholesterol Conjugate (DP7-C)
[0065] Antimicrobial peptide DP7 and hydrophobic segment conjugates are made of figure 1Synthesized by the synthetic route shown. Wherein, the hydrophobic segment includes: cholesterol, cholic acid, palmitic acid, stearic acid, lauric acid and the like. 2-chlorotrityl chloride Resin, whose name is 2-chlorotrityl chloride resin; Fmoc-Rink Amide MBHA Resin, whose name is 4-(2',4'-dimethoxyphenyl-fluorenylmethoxycarbonyl- Aminomethyl)-phenoxyacetamido-methylbenzhydrylamine resin; Fmoc: fluorenylmethoxycarbonyl; pbf, tbu, Otbu, Trt, Boc are all protecting groups, the names are 2, 2, 4, 6 , 7-pentamethyldihydrobenzofuran-5-sulfonyl, tert-butyl, tert-butoxy, trityl, tert-butoxycarbonyl.
[0066] Concrete synthesis method is as follows:
[0067] 1. Resin swelling activation and deprotection:
[0068] Swelling: Weigh 1.0g Rink MBHA (4-(2′,4′-dimethoxyphenyl-fluorenylmethoxycarbonyl-aminome...
Embodiment 2
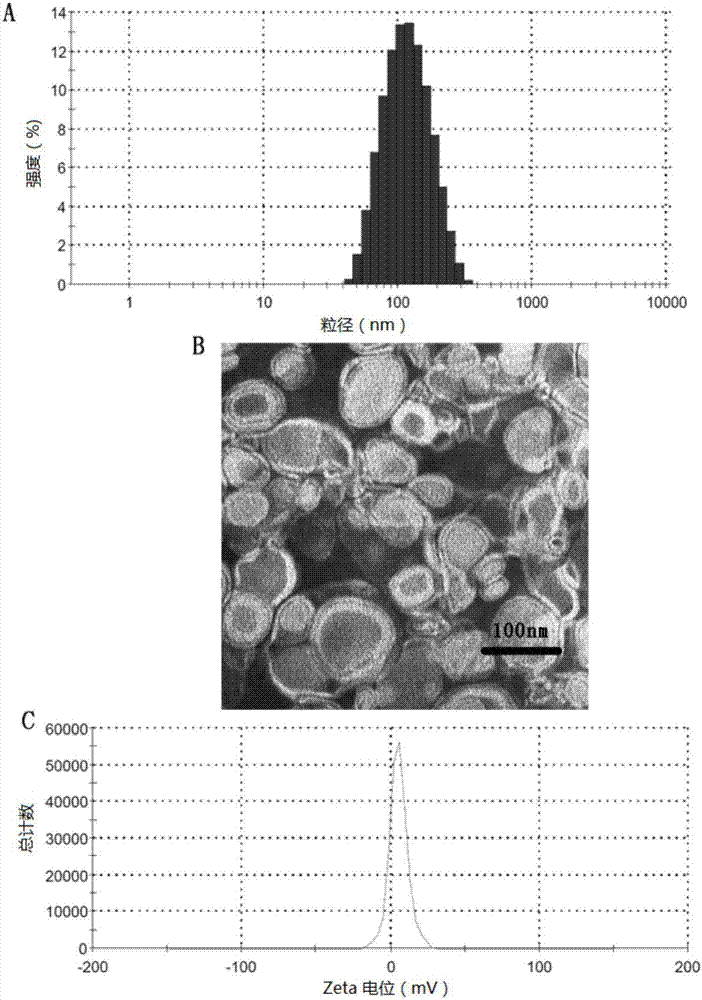
[0090] Preparation and characterization of liposomes modified by embodiment two DP7-C
[0091] 1. Liposome preparation and process optimization
[0092] Azithromycin-loaded liposomes (AZT-LPs) were prepared by thin film dispersion method.
[0093] The specific method is: accurately weigh 15.0mg soybean lecithin (SPC), 5.0mg cholesterol (Chol) (the mass ratio of lecithin and cholesterol is 3:1) and a certain amount of azithromycin are dissolved in 4mL chloroform solution; , in a 100mL round bottom flask, 37°C, 50 rpm, rotate under reduced pressure for 2h to evaporate the organic solvent to form a lipid film. Then, 4 mL of PBS solution was added to the flask, and the flask was rotated at 55°C to hydrate the lipid film for 40 min to prepare AZT-LPs. Afterwards, the above-mentioned liposome solution was sonicated with a probe at 85W power for 3 minutes (3 seconds of ultrasound, with an interval of 3 seconds), and then filtered with a 0.2 μm filter membrane to obtain a liposome s...
Embodiment 3
[0129] Embodiment three Liposome cytotoxicity detection of the present invention
[0130] Using human LO2 and HEK293 cells as model cells, Free AZT (250 μg / ml), AZT-LPs (250 μg / ml), D-LPs (250 μg / ml) and 1.25% AZT-D-LPs (62.5 μg AZT / ml+250μg DP7-C / ml), 2.5% AZT-D-LPs (125μg AZT / ml+250μg DP7-C / ml) and 5% AZT-D-LPs (250μg AZT / ml+250μgDP7-C / ml) Cell viability after 24 hours of liposome action. The cells in the logarithmic growth phase were collected, seeded in a 96-well plate at a density of 5×103 / well, and cultured in a cell culture incubator at 5% CO2, saturated humidity, and 37°C. After the cells adhered to the wall for 24 hours, 100 μl of Free AZT, AZT-LPs, D-LPs, and AZT-D-LPs solutions with different drug loadings were added to each well, and an equal volume of PBS was used as a negative control well. Concentration set up 3 parallel wells, and repeated 3 parallel experiments. After continuing to cultivate for 24 h, the mixture of drug and medium was removed, and then 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com