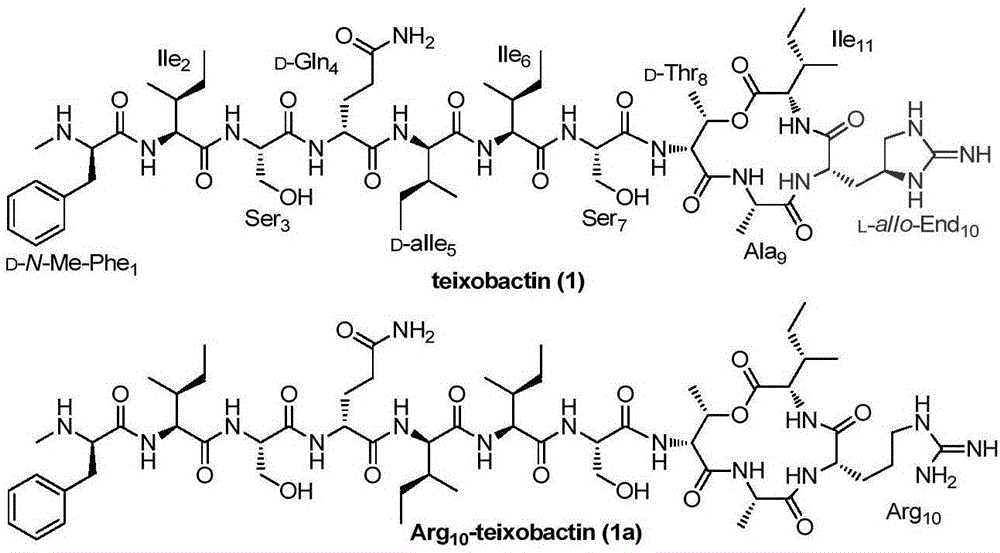
Teixobactin analogs, and preparation method and application thereof
A technology of analogues and products, applied in the field of TEIXOBACTIN analogues and its preparation, can solve the problems of many synthesis steps and low efficiency, and achieve the effect of reducing synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation method of embodiment 1 compound 1a
[0071] (1) Preparation of linear peptide:
[0072] In a 50mL solid phase reactor, add hydrazine resin (0.61mmol / g, 800mg, 0.488mmol) and CH 2 Cl 2 (3ml), swell the resin for 30min. Extract CH 2 Cl 2, 20% piperidine / DMF solution (3ml) was used to remove the Fmoc protecting group. After 10min, the resin was washed with DMF (4x3mL) and then with anhydrous DMF (2x3mL) for later use. At the same time, Fmoc-Ala-OH ((76mg, 0.244mmol) and HATU (91mg, 0.23mmol) were dissolved in anhydrous DMF (2ml), and DIEA (120μL, 0.732mmol) was added to the solution, mixed, and the reaction Liquid transfer to hydrazine resin for Fmoc removal, N 2 Bubbling and mixing, condensation reaction 2h. The reaction solution was pumped off, the resin was washed with DMF (4x3 mL), and then with anhydrous DMF (3 mL). Add a DMF (2mL) solution of pivalic anhydride (186 μL, 0.96mmol) and DIEA (500 μL, 2.88mmol) to the resin, react for 15min, remove...
Embodiment 2
[0091] The preparation method of embodiment 2 compound 1b
[0092] The preparation method of Example 2 is the same as that of Example 1, except that in the second step, Fmoc-Lys(Boc)-OH is used to replace Fmoc-Arg(Pbf)-OH. Other steps are the same.
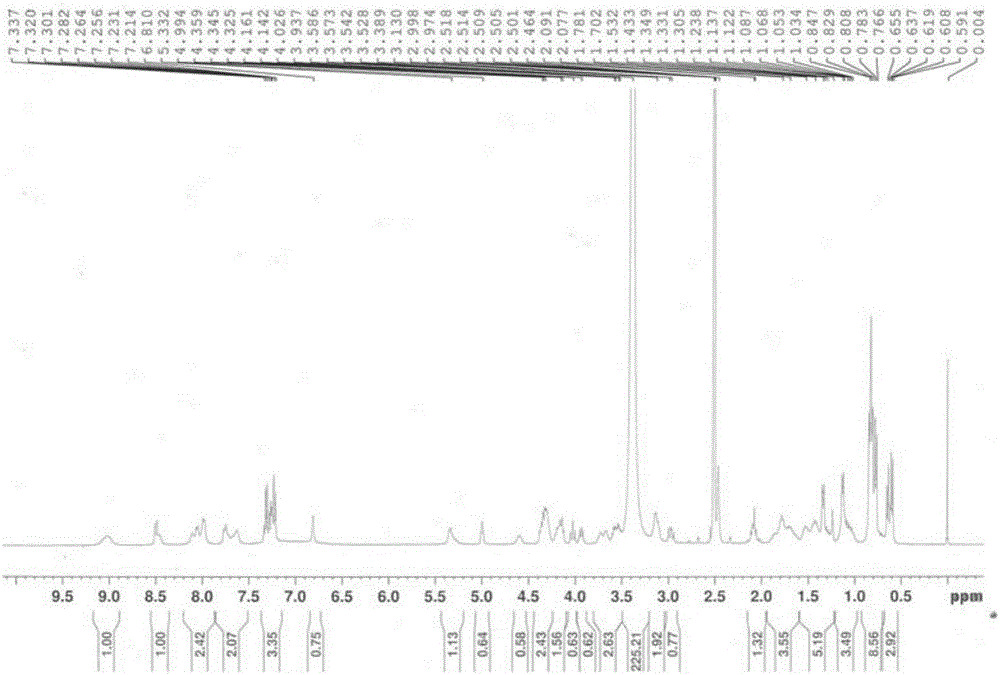
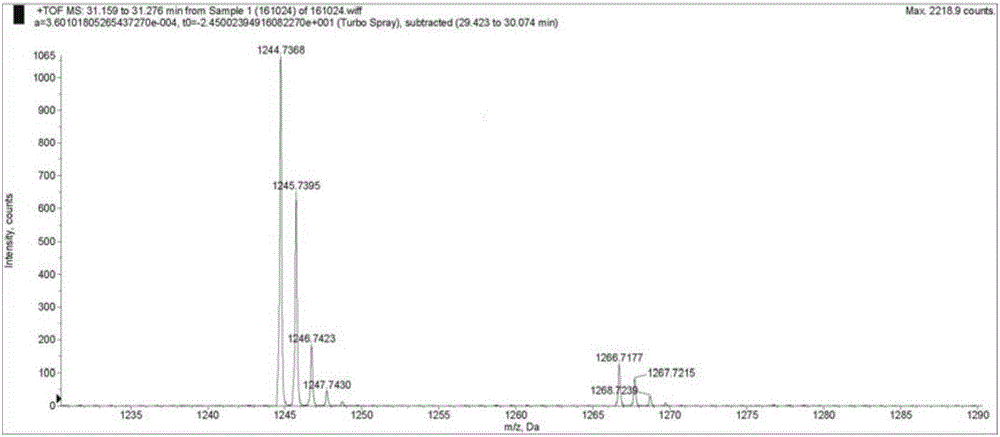
[0093] Lys 10 -teixobactin(1b): 1 H NMR (DMSO-d 6 ,400MHz):δ0.52-0.64(m,6H),0.68-0.97(m,19H),0.97-1.20(m,7H),1.20-1.47(m,8H),1.47-1.95(m,10H) ,1.95-2.19(m,2H),2.46(s,3H),2.62-2.89(m,2H),2.89-3.01(m,1H),3.02-3.18(m,1H),3.49-3.78(m, 4H),3.87-4.08(m,2H),4.08-4.50(m,9H),4.50-4.70(s,1H),4.87-5.13(s,1H),5.20-5.45(m,2H),6.70- 6.88(s,1H),7.11-7.38(m,5H),7.50-7.85(m,6H),7.88-8.20(m,5H),8.30-8.59(m,2H),8.92-9.30(m,2H ) ppm; HRMS (ESI) m / z: calcd for C 58 h 98 N 13 o 15 [M+H] + 1216.7305,found 1216.7280.
Embodiment 3
[0094] The preparation method of embodiment 3 compound 1c
[0095] The preparation method of Example 3 is the same as that of Example 1, except that in the second step, Fmoc-His(Trt)-OH is used to replace Fmoc-Arg(Pbf)-OH. Other steps are the same.
[0096] His 10 -teixobactin(1c): 1 H NMR (DMSO-d 6 ,400MHz):δ0.50-0.69(m,6H),0.69-0.91(m,16H),0.95-1.35(m,11H),1.35-1.59(m,3H),1.61-2.00(m,5H) ,1.95-2.20(m,2H),2.46(s,3H),2.90-3.20(m,4H),3.49-3.77(m,5H),3.82-3.92(m,1H),3.95-4.03(m, 1H),4.09-4.28(m,3H),4.28-4.43(m,3H),4.51-4.78(m,2H),4.90-5.10(m,1H),5.15-5.40(m,2H),6.71- 6.85(m,1H),7.15-7.37(m,5H),7.70-7.90(m,2H),7.90-8.19(m,5H),8.32-8.57(m,2H),8.87-8.79(s,1H ) ppm; HRMS (ESI) m / z: calcd for C 58 h 92 N 14 o 15 [M+H] + 1225.6945,found 1225.6944.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com