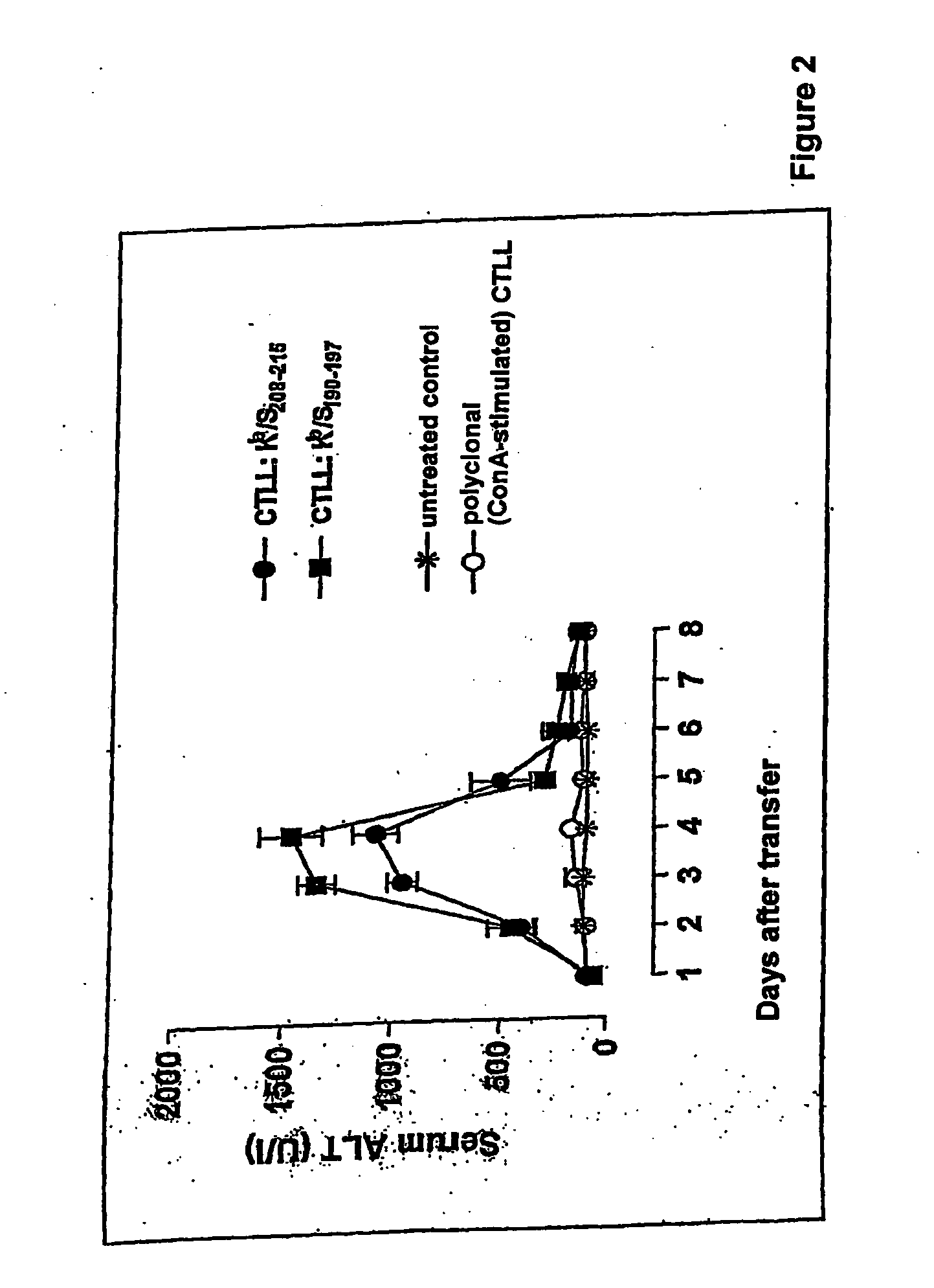
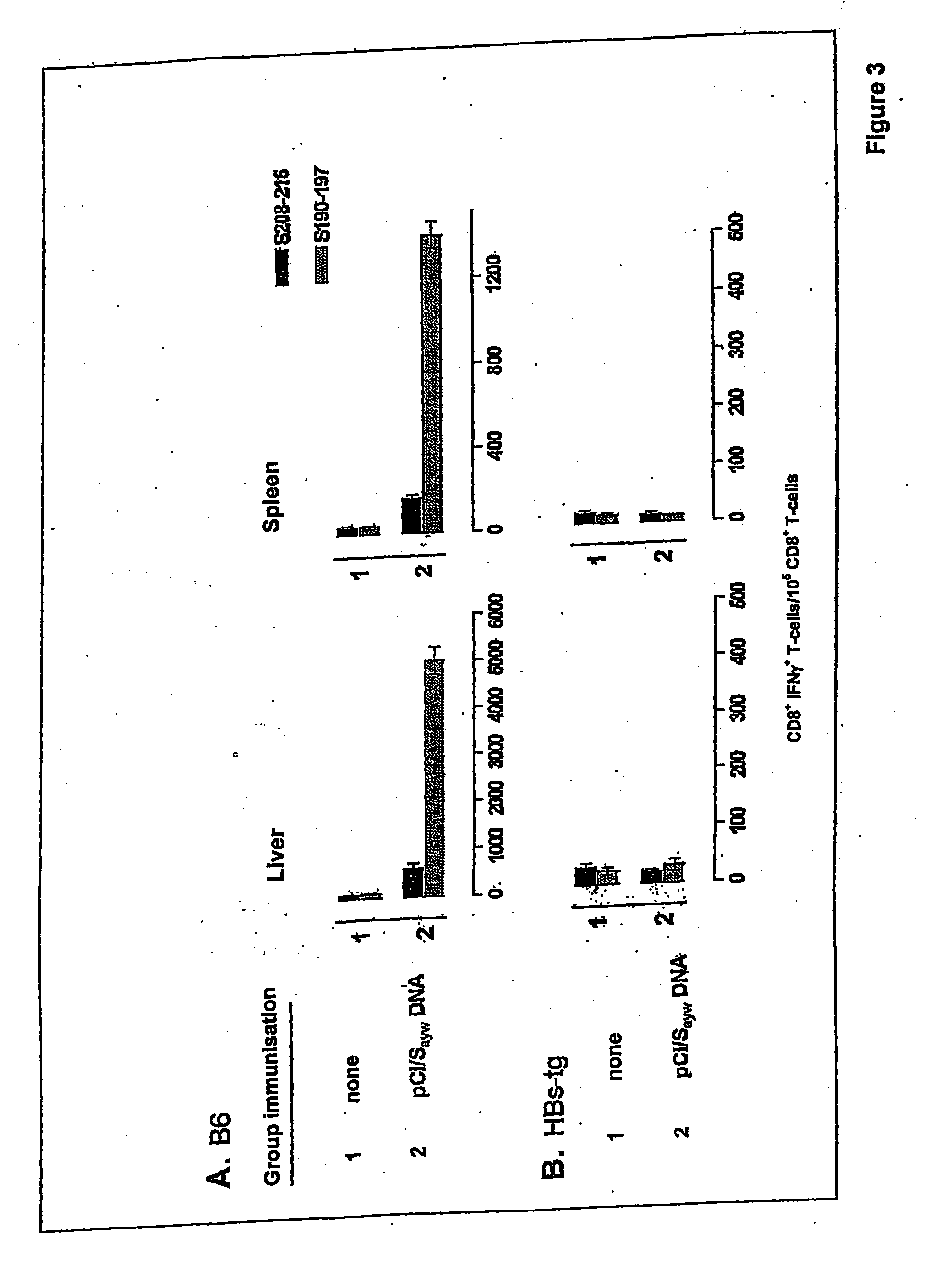
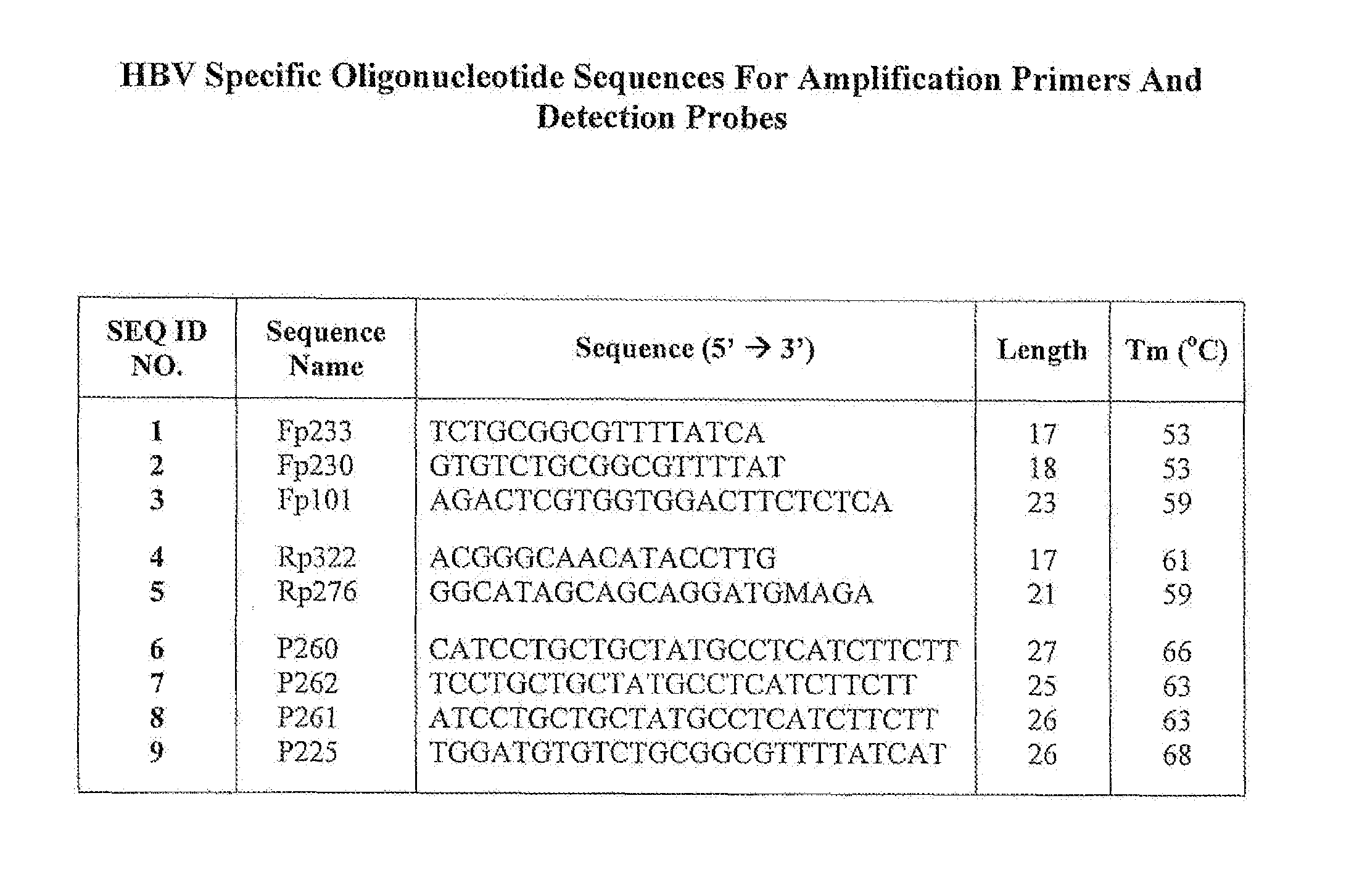
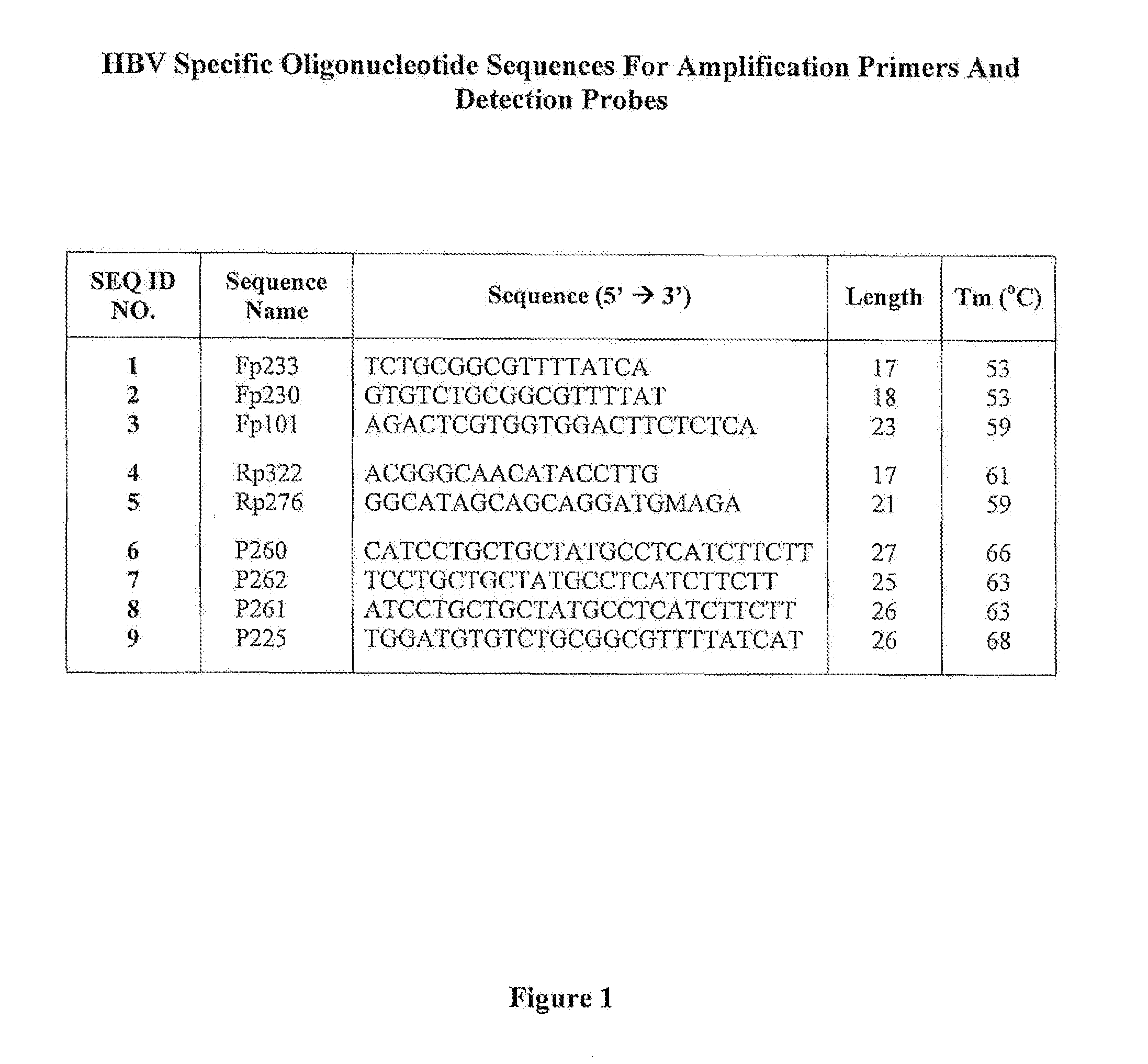
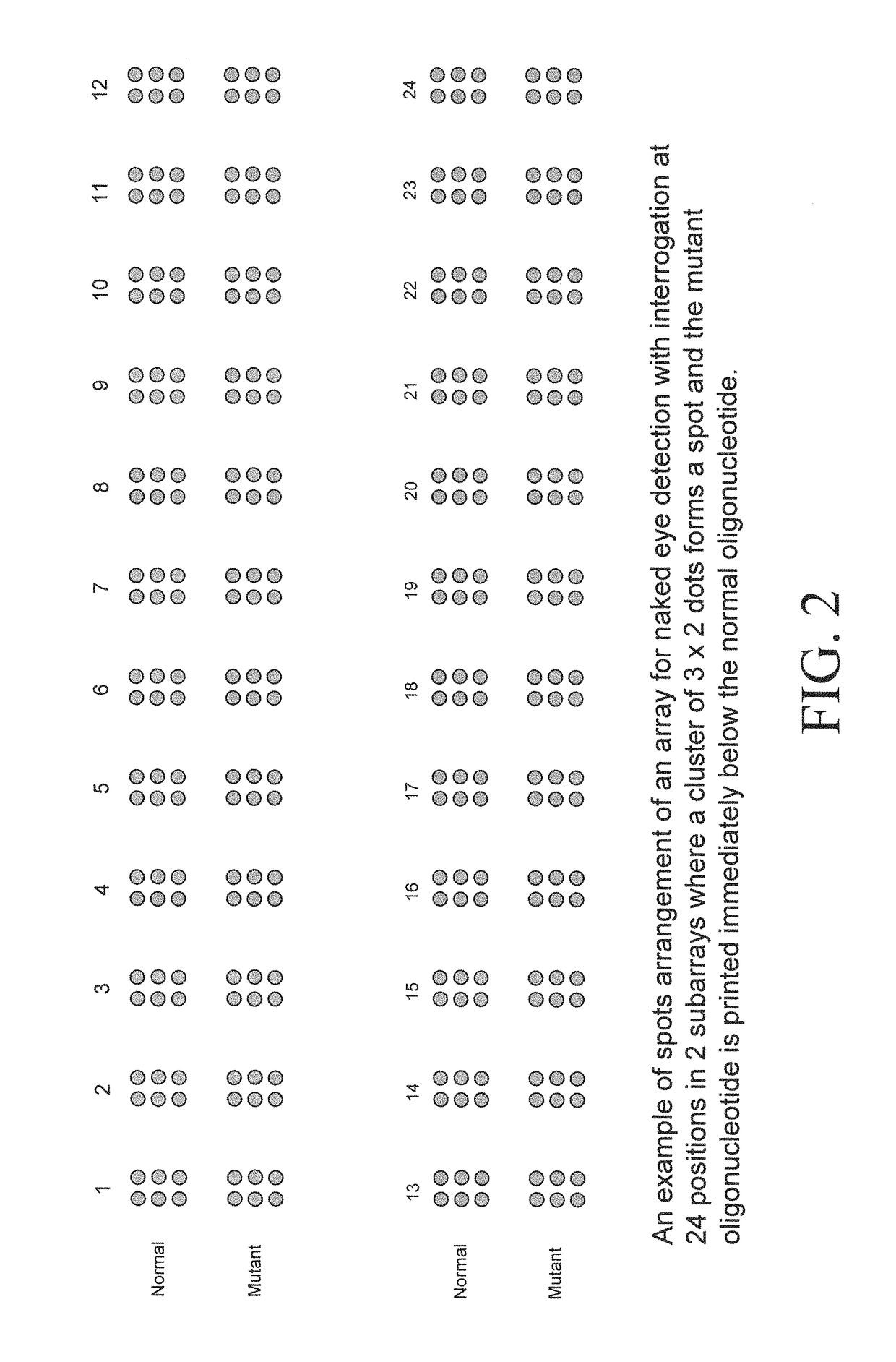
Patents
Literature
38 results about "Hbv genotype" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
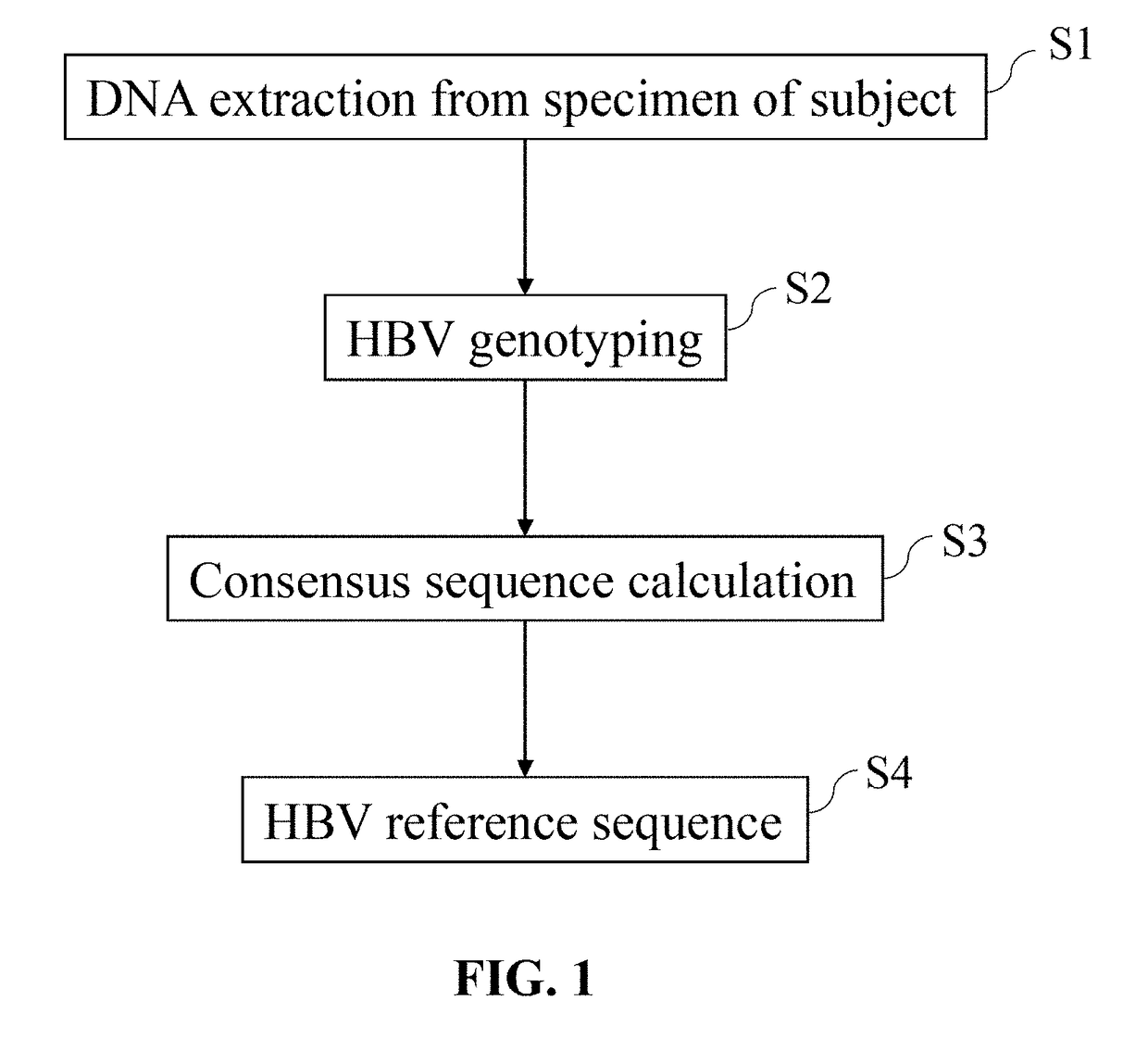
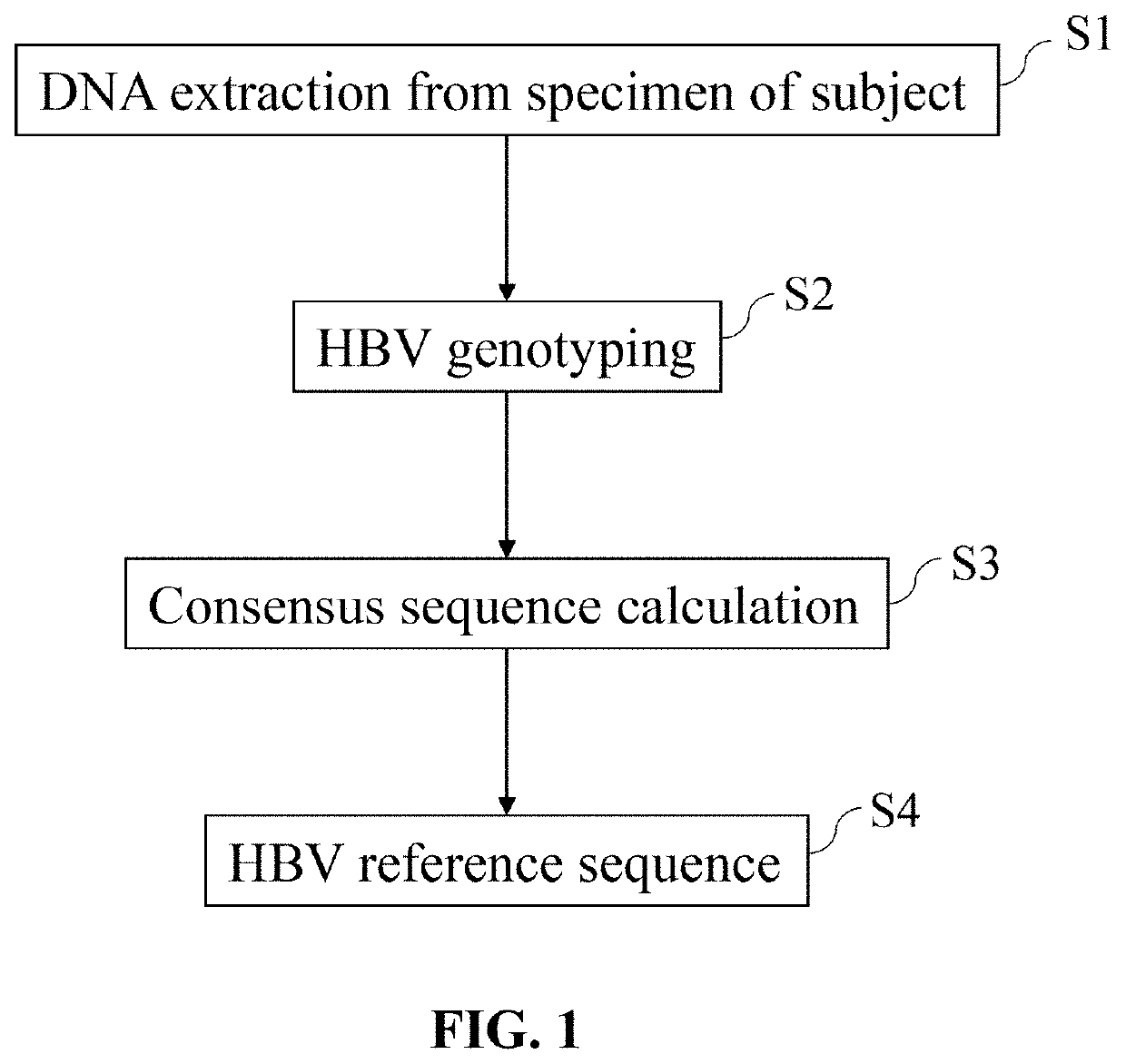
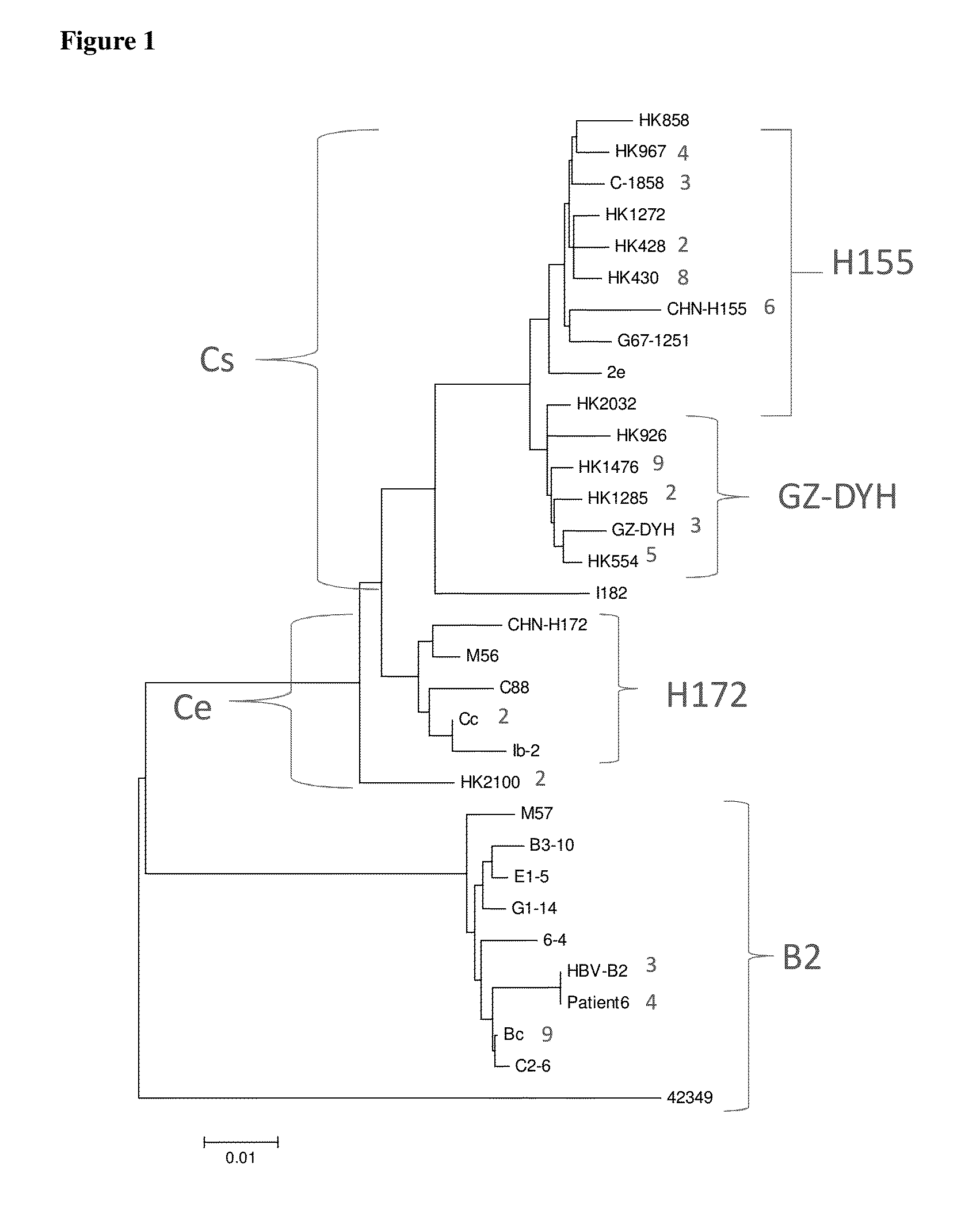
Hepatitis B virus (HBV) variants belong to different genotypes, A-J, whose worldwide distribution is linked with geography, probably because viral spread was associated with ancient human migrations. HBV genotype G (HBV-G) is an aberrant genotype with little sequence divergence, suggesting a recent origin.
Composition for the prophylaxis and treatment of HBV infections and HBV-mediated diseases
InactiveUS20060233832A1Easy to handleSimple preparation processAntipyreticGenetic material ingredientsAntigenGenotype
The present invention is a composition that comprises at least two hepatitis B virus surface antigens (HBsAgs), fragments thereof and / or nucleic acids encoding them, the HBsAgs differing in HBV genotype in the S region and / or pre-S1 region and the composition containing no HBV core antigen (HBcAg) or nucleic acid encoding that antigen. The present invention also includes pharmaceutical compositions, especially vaccines, comprising these compositions for the prevention and / or treatment of an HBV infection or an HBV-mediated disease. The present invention further includes a method of preparing a patient-specific medicament for the therapeutic treatment of hepatitis B.
Owner:RAJN BIOTEKH GEZELLSHAFT FJUR NOJE BIOTEKHNOLOGISHE PROTSESSE & PROD MBKH
Hepatitis B Virus (HBV) Specific Oligonucleotide Sequences
InactiveUS20100255482A1Wide rangeSugar derivativesMicrobiological testing/measurementOligonucleotide primersHbv genotype
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Fluorescent PCR detecting method for hepatitis B virus gene parting and reagent kit
ActiveCN1588066AShorten the experimental processShort timeMicrobiological testing/measurementBiological testingSequence analysisType specific
The invention relates to a fluorescent PCR detecting method for a HBV type seperating and the reagent box. The characteristics of the method lies in finding out the HBV DNA complete sequence which has been seperatedd from the GenBank to make a sequence; according to the result of the sequence matching, finding out the gathering zone of the type specific base of the BHA gene type, designing a probe and a pair of primers according the gathering zone. Extract HBV DNA from the blood serum sample to make PCR amplification to realize HBV gene type seperating detection in the fluorescent PCR when the probe and primer exist. The invention provides a reagent box and the type positive standard used in it. The accuracy of type parting reaches to 98% and can reach to 100% by adjusting the reaction condition., simpler and quicker, the test procedure and the time consuming is less, the cost is lower than the complete sequence analysis and more quick and accurate than the regular relevant PCR and RFLP method, besides, the closed check adopted in the invention can avoid the contamination efficiently.
Owner:GUANGZHOU HUAYIN MEDICINE SCI & TECH
HBV DNA gene subtype detecting method and kit
InactiveCN101045939ASimplified setup requirementsSimplify protection requirementsMicrobiological testing/measurementFluorescenceHbv genotype
The present invention relates to HBV DNA gene subtype detecting method and kit. Specific probe and matched primer or specific primer and matched reverse primer and specific virus detecting probe are designed based on HBV genotype characteristic sites for detecting HBV genotype alone or performing real-time fluoroscopic examination on the identical sample in several PCR tubes to detect type B and type C or general type. Through the detection, Ct difference is detected and the middle virus content in clinical HBV sample is judged. The kit of the present invention has stable performance, simple operation, fast detection and capacity of fast judging HBV DNA type, and is used in the HBV antagonizing treatment and prognosis analysis.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
HBV DNA gene parting fluorescence PCR multicenter detecting method and kit thereof
InactiveCN101195843ASimplified setup requirementsSimplify protection requirementsMicrobiological testing/measurementFluorescenceType specific
The invention relates to a HBV-DNA gene typing fluorescence PCR multichannel detection method and the reagent box thereof. The method is characterized in that a HBV-DNA full sequence adopting the gene typing is found out of GenBank, and then sequence alignment is performed to the HBV-DNA full sequence; a gathering area of a type specific base of an HBV genotype is found out according to the sequence alignment result; a probe and the matched primer thereof are designed according to the gathering area of the type specific base, multichannel genotype detection and general type detection are performed in a PCR pipe through adopting a multicolour marking probe, and according to the difference value of the Ct value obtained through the multichannel genotype detection and general type detection, the proportion content of the HBV virus medium virus in the clinical samples is judged. The reagent box of the invention has the advantages that the property is stable, the operation is simple and convenient, the detection is quick, the type of the HBV-DNA can be perfectly judged, and the reagent box is in favor of judging the prognosis of chronic hepatitis B virus infected persons more comprehensively and choosing more appropriate therapeutic schedule.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Hepatitis b virus typing and resistance assay
ActiveUS20130252232A1Confer resistanceMicrobiological testing/measurementReverse transcriptaseNucleotide
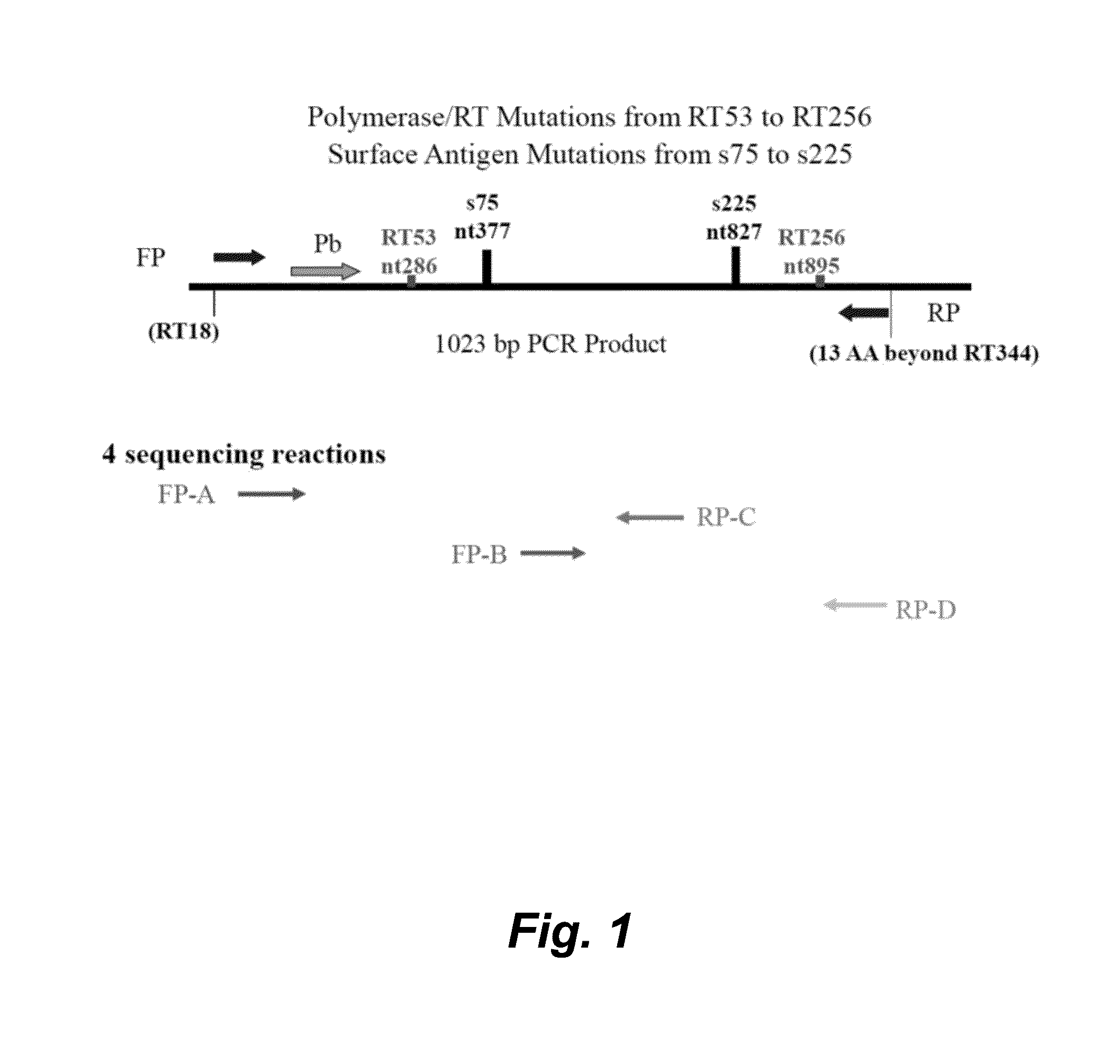
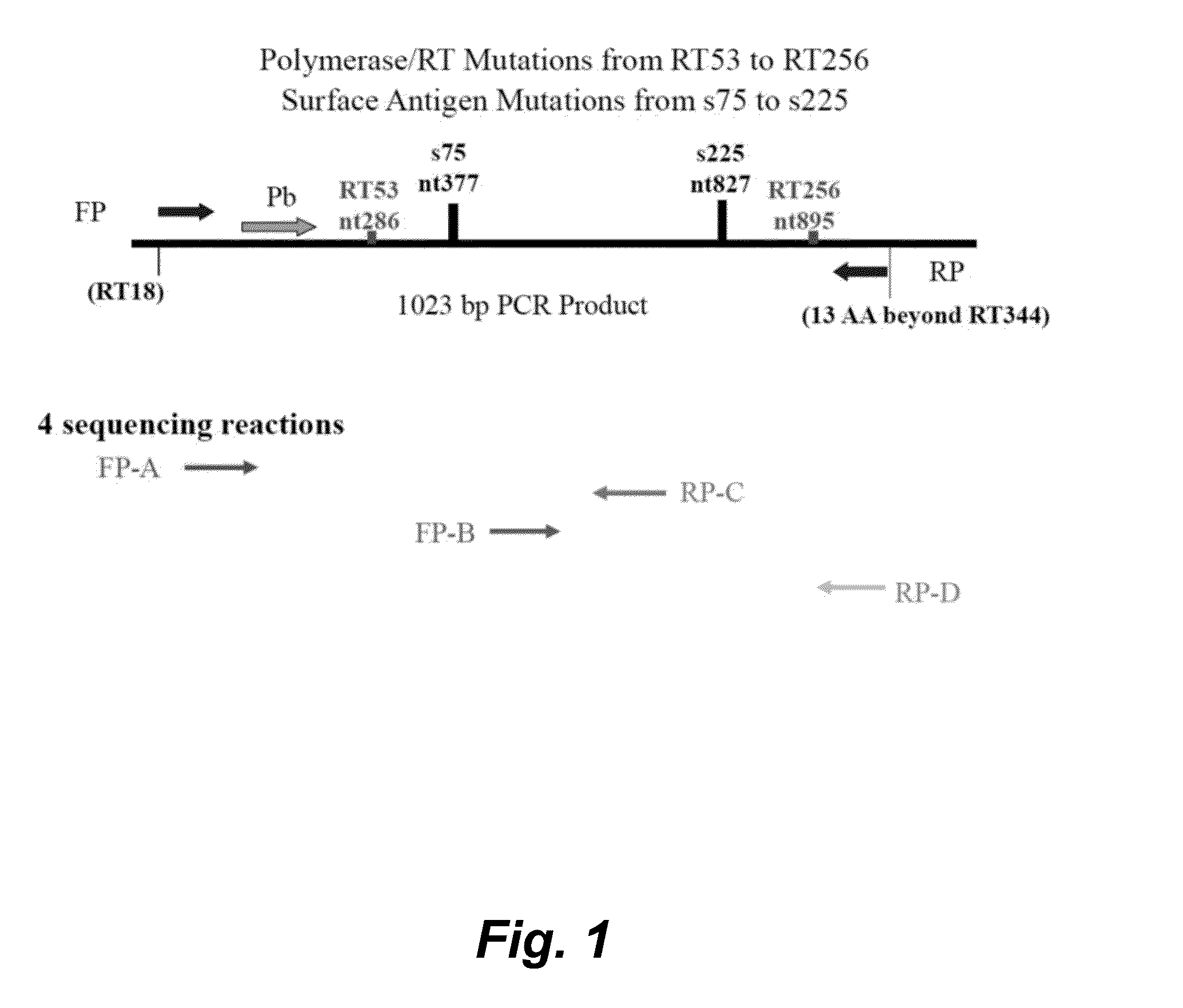
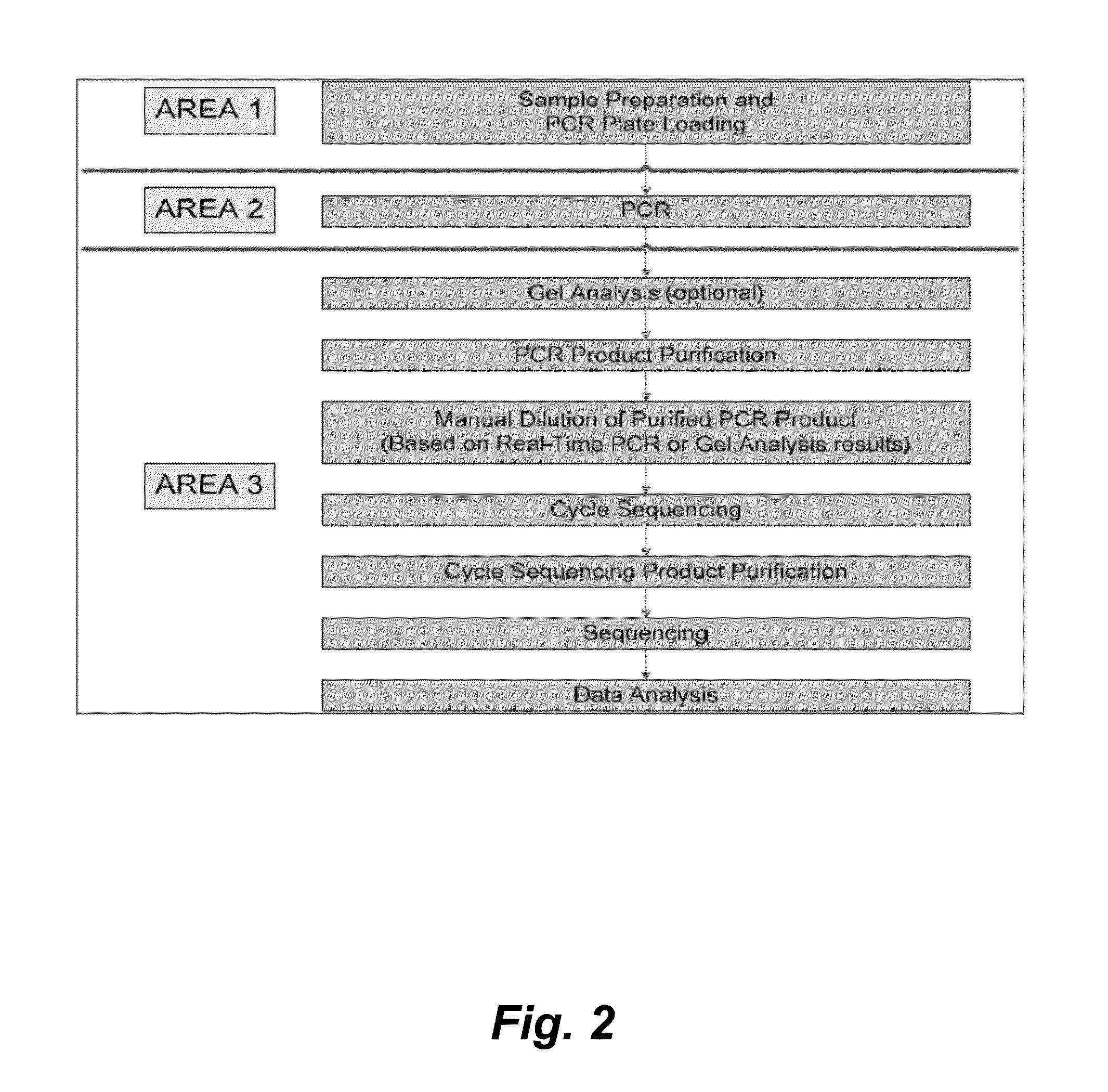
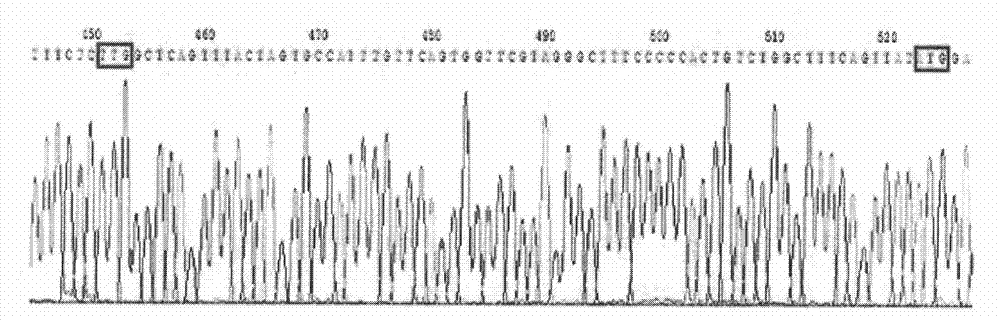
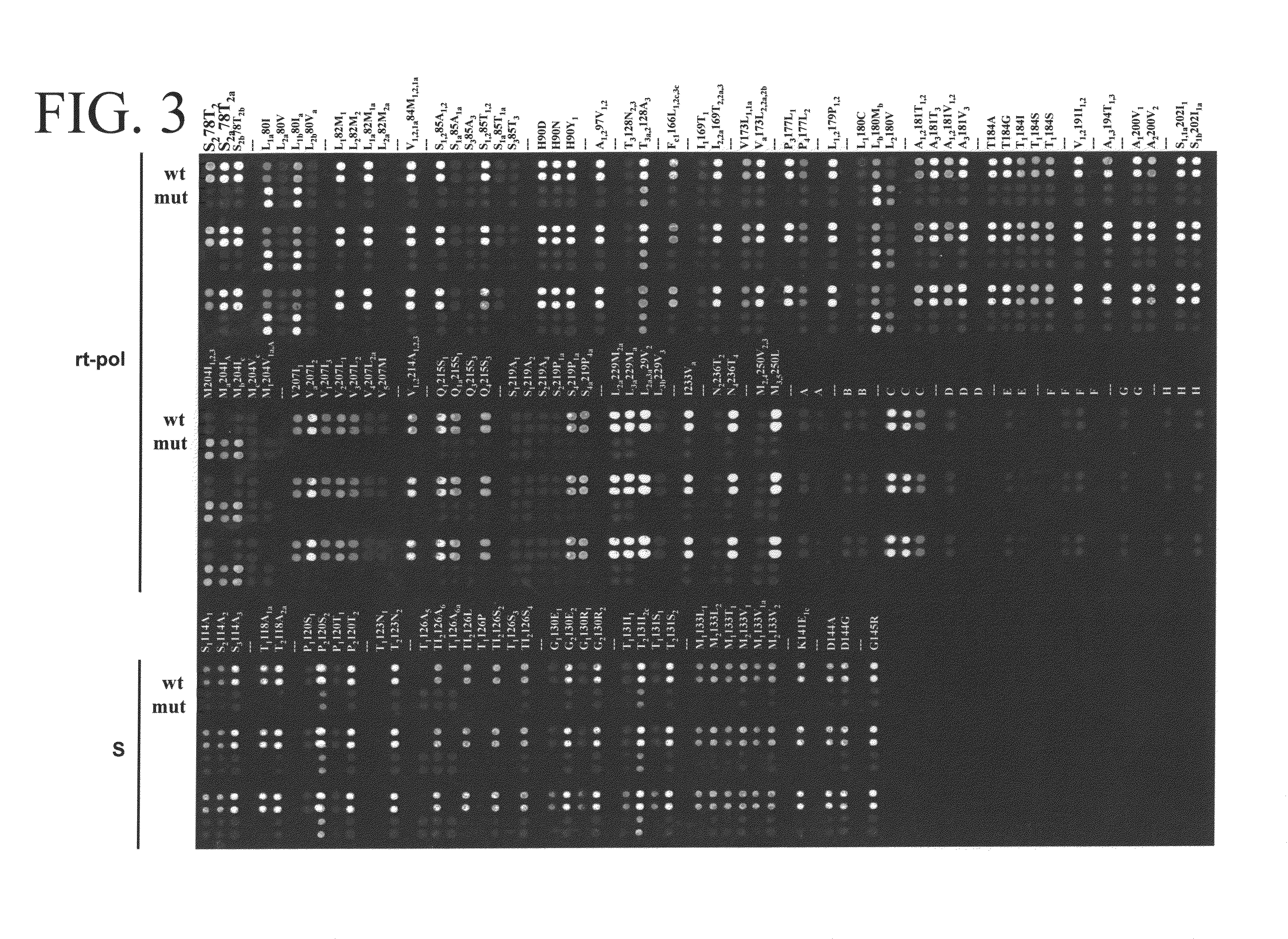
The present invention provides methods, kits, and oligonucleotides for detecting and analyzing the nucleotide sequence of a reverse transcriptase (RT) region of the polymerase (Pol) gene of Hepatitis B Virus (HBV). In certain embodiments, a target RT region is amplified and subjected to DNA sequencing. The sequence obtained is compared to one or more DNA sequences characteristic of an HBV genotype or serotype, and / or one or more DNA sequences characteristic of an HBV mutation that confers resistance to a drug or vaccine, to determine the HBV genotype or serotype of the amplified product and / or the presence or absence of one or more DNA sequences characteristic of an HBV mutation that confers resistance to a drug or vaccine.
Owner:ABBOTT MOLECULAR INC
Polymerase chain reaction detection method of hepatitis B virus genotyping
ActiveCN102140537ASimple and fast operationHigh compliance rateMicrobiological testing/measurementFluorescence/phosphorescenceSerum samplesFluorescence
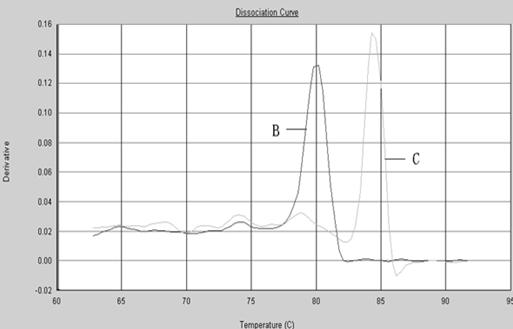
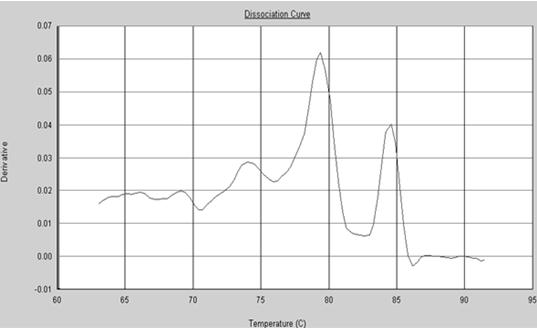
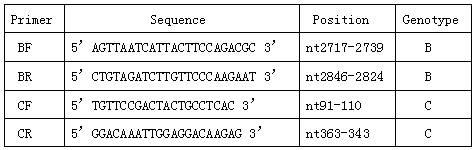
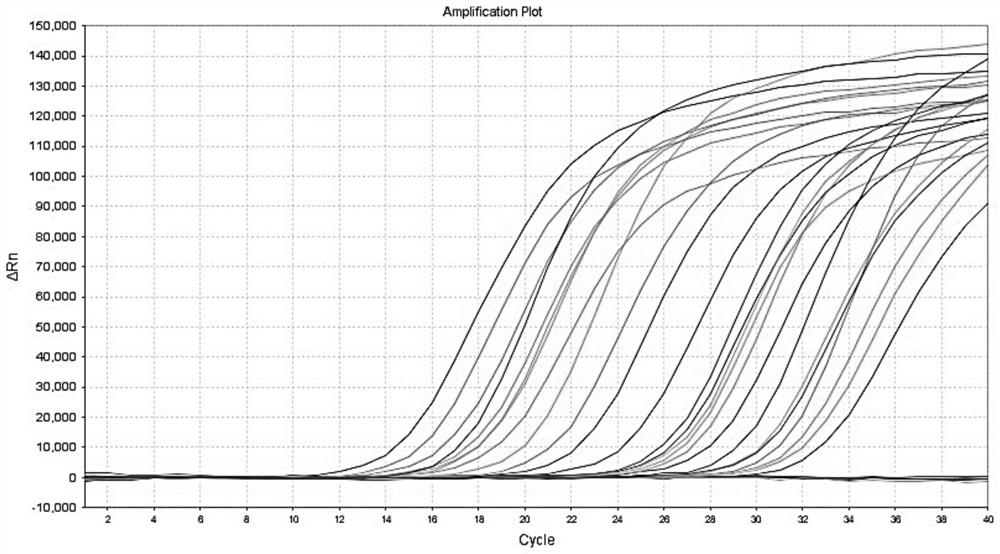
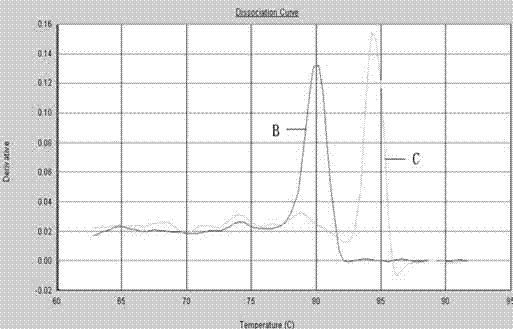
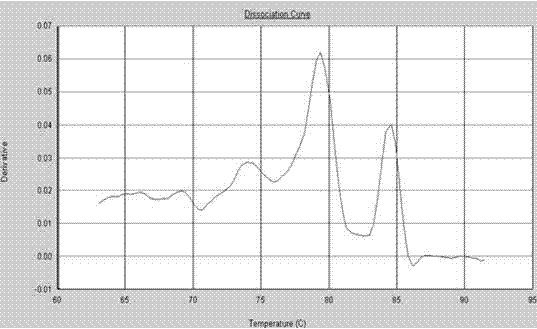
The invention provides a polymerase chain reaction (PCR) detection method of hepatitis B virus (HBV) genotyping. The method comprises the following steps: a primer is designed according to the entire genome sequence of the HBV genotype; in the existence of the primer, DNAs are extracted from a serum sample to perform PCR amplification in a fluorescence PCR meter, the PCR product is analyzed according to the melting curve, the genotype is judged according to the Tm value of the PCR product and the HBV genotyping can be realized. The PCR melting curve method used in the invention is convenient to operate, the detection result of the method has higher coincidence rate with the detection result obtained by adopting a commercial genotyping kit; and the method can be used in the clinical detection of the HBV genotype. On the basis of the PCR technology, the PCR melting curve method which is to judge the HBV genotype according to the Tm value, is provided in the PCR detection method, thus the HBV genotype can be identified conveniently, rapidly and accurately.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
HBV YMDD mutation, precore region mutation/BCP region mutation and genotyping integrated-detection DNA microarray chip
InactiveCN101660000AImprove throughputImprove accuracyMicrobiological testing/measurementDNA Microarray ChipHbv genotype
The invention relates to a DNA microarray chip, in particular to an HBV YMDD mutation, precore region mutation / BCP region mutation and genotyping integrated-detection DNA microarray genetic chip. A solid-phase carrier substrate is used, and an oligonucleotide probe is designed in view of the HBV YMDD mutation, the precore region / BCP region mutation sites and the HBV genotype so as to prepare the DNA microarray chip. The DNA microarray chip can simultaneously detect three indexes at one time and can simultaneously detect a plurality of samples. The DNA microarray chip can be widely used to individual medicine taking guidance in the clinical process of treating HBV infections.
Owner:DAAN GENE CO LTD
Method and kits for detecting genotype of hepatitis B virus
InactiveCN101586170AHigh mutation rateAvoid missing detectionMicrobiological testing/measurementMicroorganism based processesCross-linkConserved sequence
The invention provides a method and kit for detecting genotype of hepatitis B virus, the method includes following steps: 1, extracting DNA genome of HBV from blood serum or blood plasma; 2, designing typing premer and probe according to conserved sequence of HBV genome; 3, using micromolecule marked oligonucleotide primer to proceed PCR reaction to amplify target DNA sequence; 4, oligonucleotide probe is added with a tail chain poly dC, then UV cross-linking on substrate and baking at a temperature of 120 DEG for fixing; 5, hybridizing amplication product of the marked target DNA with specific oligonucleotide probe on substrate; 6, detecting bybridization conjugates to judge different genotypes of HBV. The invention designs specific probe with high conservative and PCR amplication primer in different base position, and improves detection sensitivity, such that HBV genotypes can be detected quickly, conveniently and accurately.
Owner:CHONGQING MEDICAL UNIVERSITY
Gene-sequencing-method-based hepatitis B virus genotyping kit
InactiveCN102181584AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementMicroorganism based processesTherapeutic effectGenotype
The invention relates to the field of biotechnology. At present, determined genotypes of hepatitis B virus (HBV) comprise eight types A, B, C, D, E, F, G and H, and the pathogenicity, the medicine resistance and the treatment effects of antiviral medicaments vary with the genotypes. In order to identify the HBV genotypes of clinical patients and patients to be tested, the invention provides the gene-sequencing-method-based hepatitis B virus genotyping kit, and a method for identifying the genotypes comprises the following steps of: (1) extracting an HBV deoxyribonucleic acid (DNA) genome fromserum or blood plasma; (2) designing genotyping primers according an HBV S area; (3) performing amplification on a target DNA fragment by a polymerase chain reaction (PCR); (4) detecting a gene sequence of the target DNA by a gene sequencing instrument; and (5) judging the genotype of HBV according to the gene sequence of the target DNA of the HBV. The kit is accurate in result and high in sensitivity, can be operated with high flux, is convenient to popularize and use, and provides a basis for the clinical treatment and rational administration of medicaments for HBV patients.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis b virus typing and resistance assay
InactiveUS20150259755A1Peptide/protein ingredientsMicrobiological testing/measurementNucleotideReverse transcriptase
The present invention provides methods, kits, and oligonucleotides for detecting and analyzing the nucleotide sequence of a reverse transcriptase (RT) region of the polymerase (Pol) gene of Hepatitis B Virus (HBV). In certain embodiments, a target RT region is amplified and subjected to DNA sequencing. The sequence obtained is compared to one or more DNA sequences characteristic of an HBV genotype or serotype, and / or one or more DNA sequences characteristic of an HBV mutation that confers resistance to a drug or vaccine, to determine the HBV genotype or serotype of the amplified product and / or the presence or absence of one or more DNA sequences characteristic of an HBV mutation that confers resistance to a drug or vaccine.
Owner:ABBOTT MOLECULAR INC
Probe combination for detection of cancer
ActiveUS20180223380A1Detect presenceHighly sensitive, efficient, and reliableMicrobiological testing/measurementGene targetsHbv genotype
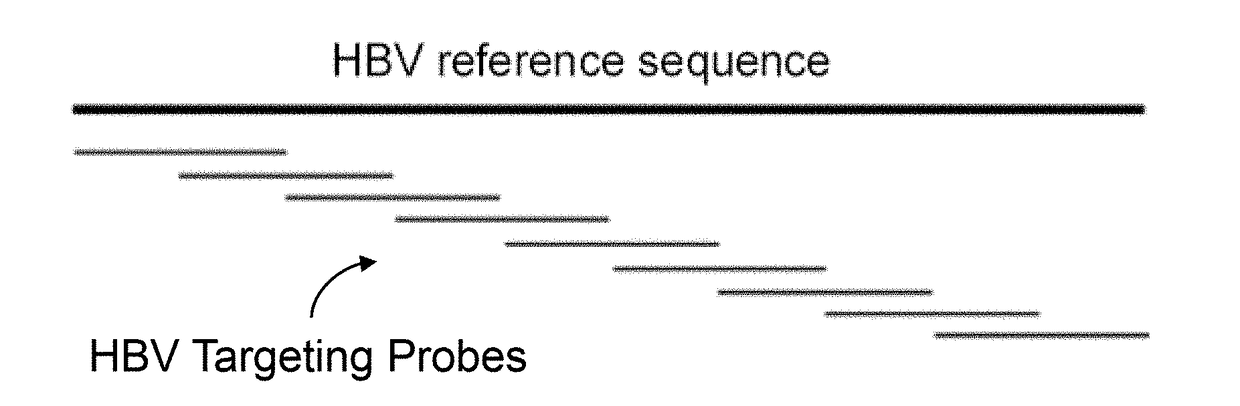
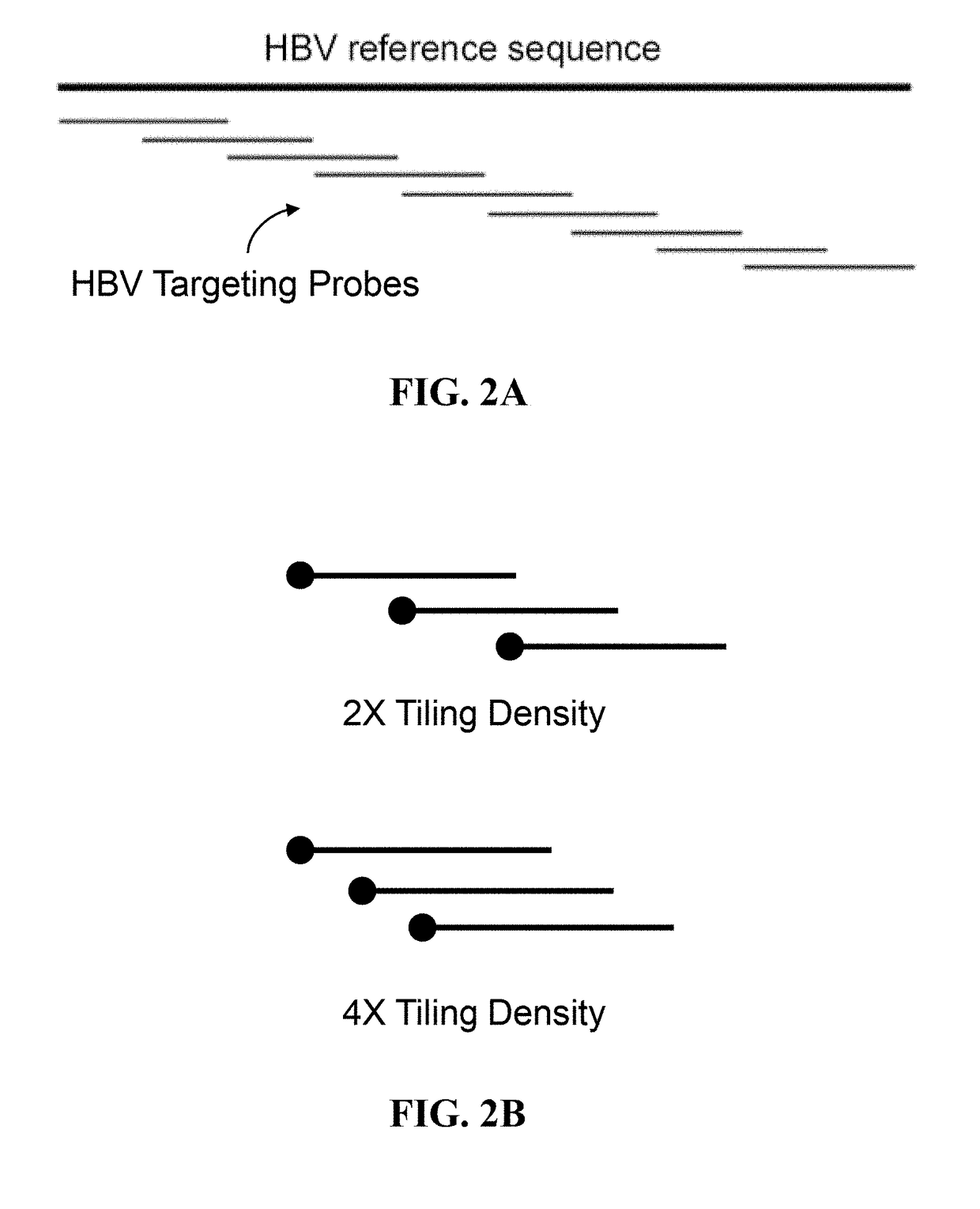
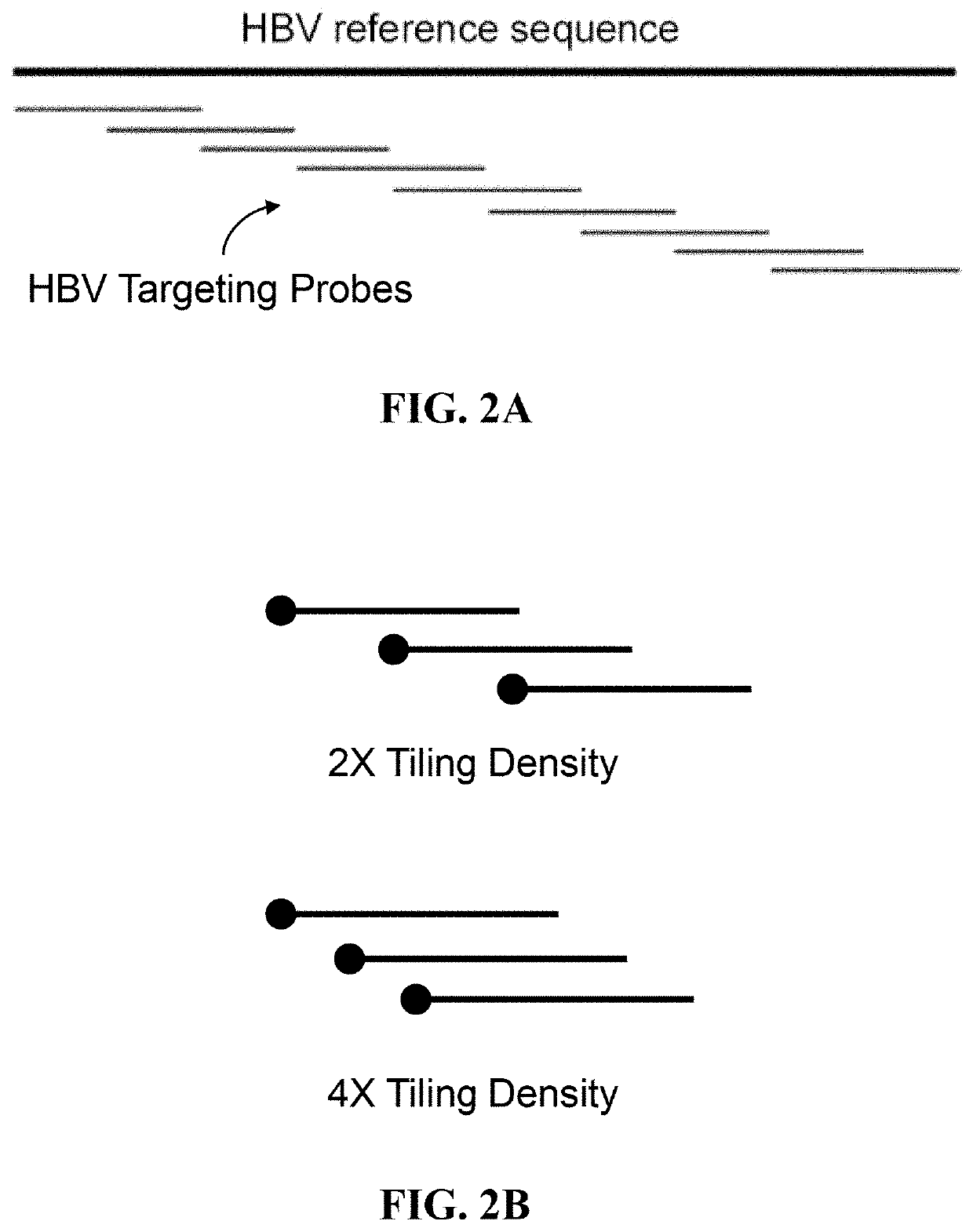
A probe combination for detecting cancer includes one or more sets of partial hepatitis B virus (HBV) targeting probes. When sequences of each of the sets of partial HBV targeting probes are aligned, an overall sequence of the aligned set of probes matches a reference sequence of a genome of a HBV genotype or a direct repeat (DR) region on the genome. In the aligned set of probes, each of the probes overlap with one or two adjacent probes by a portion of a length of the probe. The probe combination may further includes one or more sets of hotspot gene targeting probes targeting cancer hotspot genes such as CTNNB1, TERT, and TP53 genes, one or more sets of exogenous gene targeting probes targeting portions of a lambda phage genome, and endogenous gene targeting probes targeting endogenous genes such as GAPDH and GdX genes.
Owner:TCM BIOTECH INT CORP
Detection probe, detection kit and detection method for hepatitis B virus drug resistance gene mutation
InactiveCN102286645AEasy to operateStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationResistant genesMutation detection
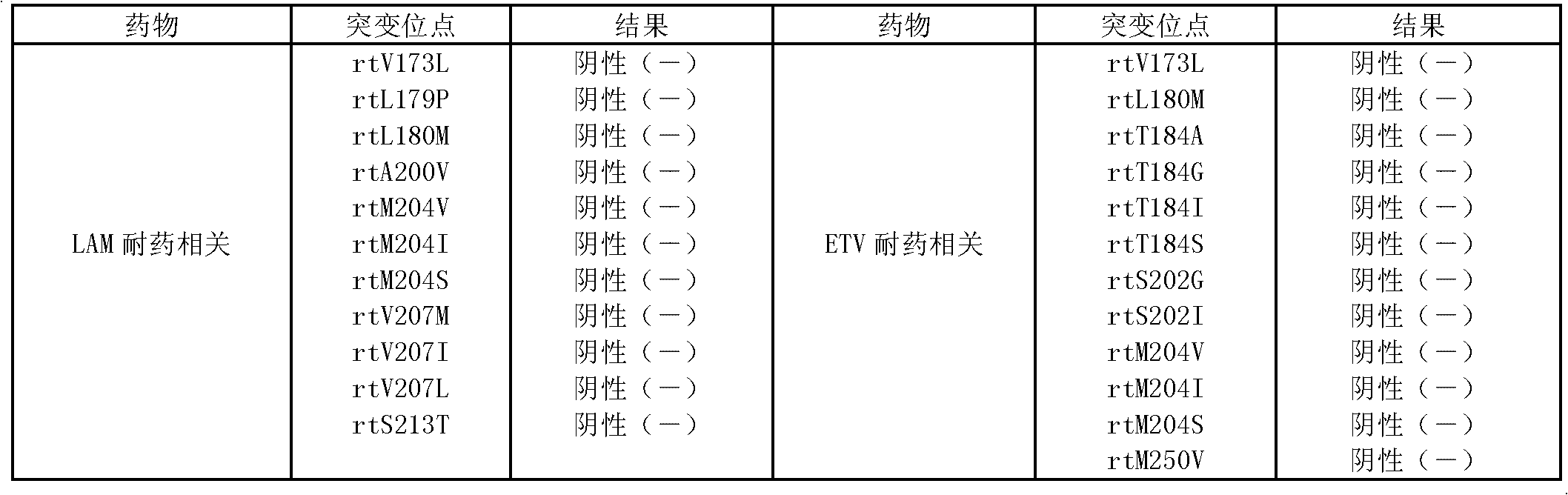
The invention provides a method for detecting mutation sites of multi-drug resistance genes of hepatitis B virus, a detection probe and a detection kit. The present invention can detect the mutation of the drug-resistant gene of hepatitis B virus very conveniently, and can detect the 18 mutant positions of the drug-resistant gene of hepatitis B virus currently disclosed by using one pair of PCR amplification primers and one detection probe any one or several of the points. Not only is the specificity good, but all mutation site detection can be completed in one detection process, which is convenient to operate, avoids many uncertain factors in multiple operations, and can greatly improve the detection accuracy. In addition, the detection method provided by the present invention can not only detect the mutation of the hepatitis B virus drug resistance gene, but also detect the genotyping of the hepatitis B virus in the same operation, with simple operation and high efficiency.
Owner:解码(上海)生物医药科技有限公司
Sucrose-DNA compound, its preparation method, related kit and method for detecting different HBV types by cooperating with glucometer
InactiveCN103224997ANovel signal acquisition methodLow costMicrobiological testing/measurementMicroorganism based processesSucroseMain channel
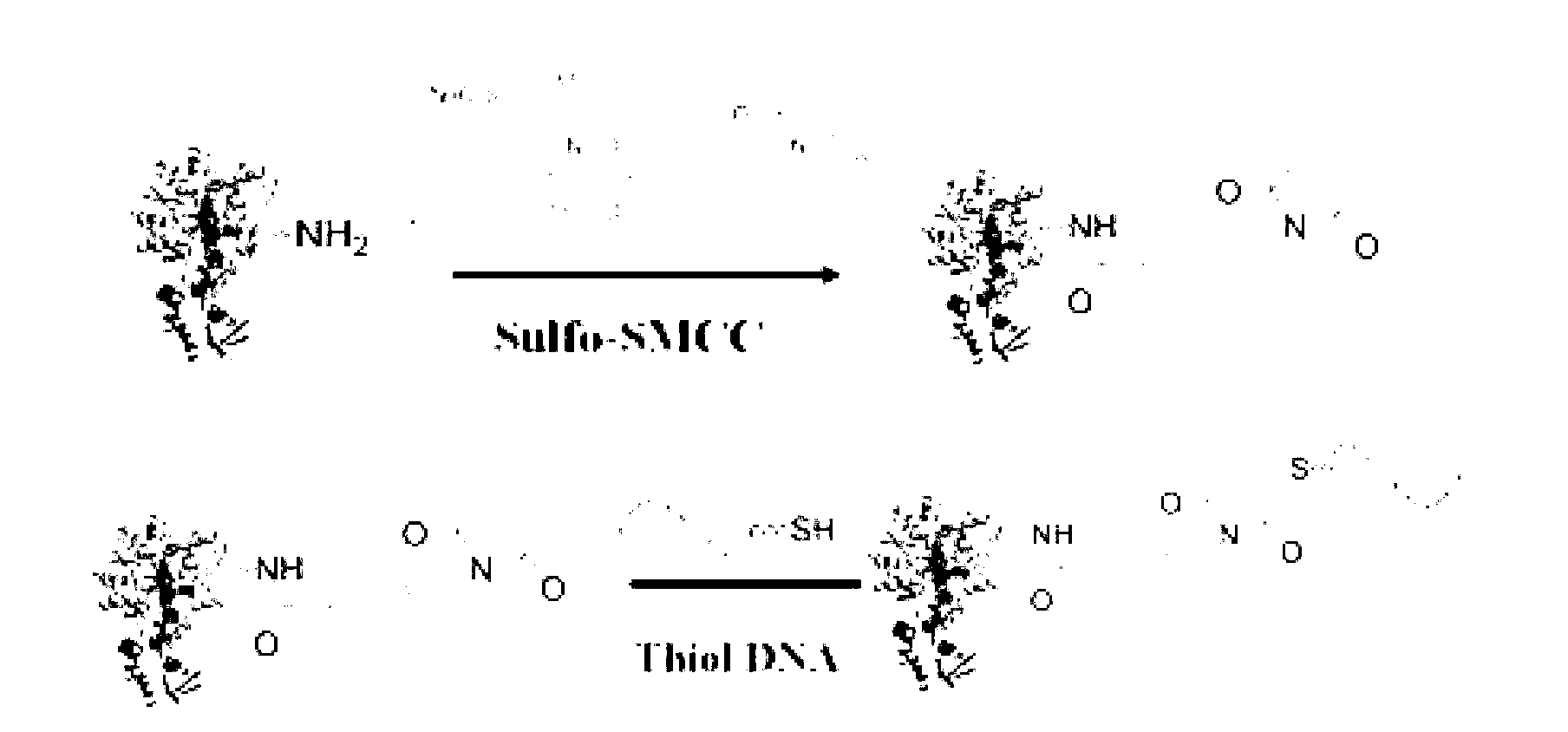
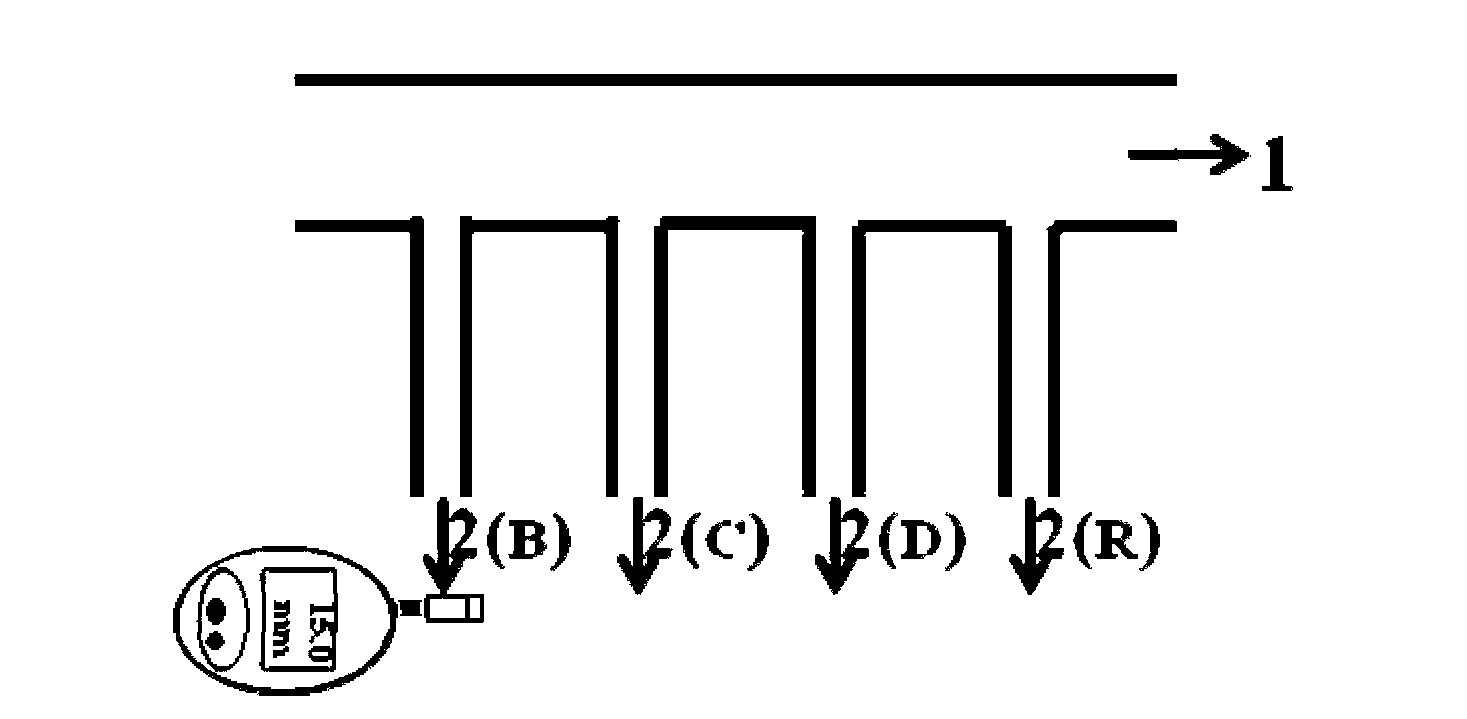
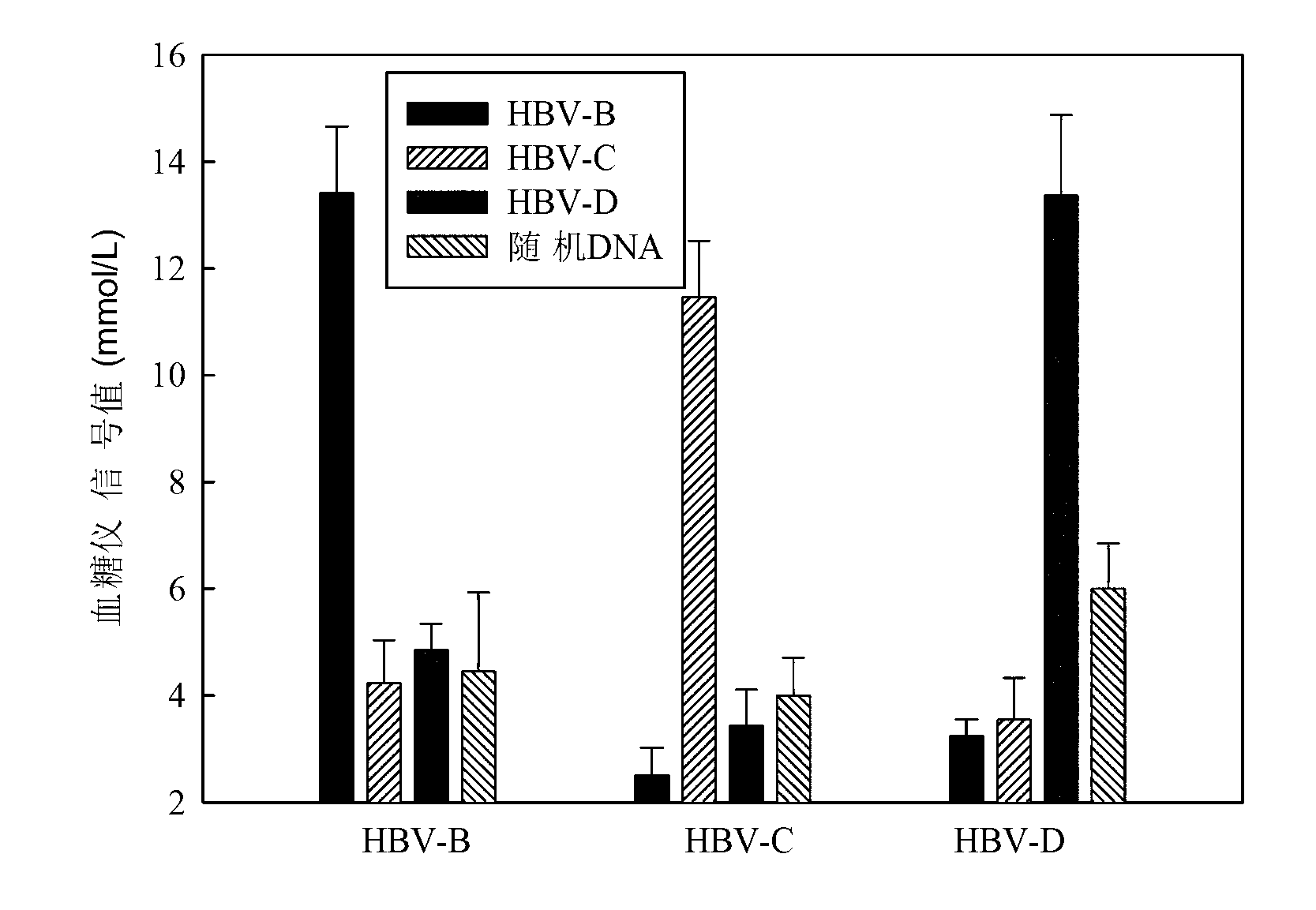
The invention discloses a sucrose-DNA compound, one end of its molecular is amino-containing sucrose, and the other end is an auxiliary probe hybridisable on different HBV genotypes. The sucrose and the auxiliary probe are connected by a connecting arm, which includes a 4-(N-maleimidomethyl)cyclohexane group. The kit provided in the invention comprises a microfluidic chip and a reporter probe solution. The chip is internally provided with a main channel and a plurality of side channels, and the different side channels are internally fixed with capture probes directed at different HBV types. The report probe solution is prepared from the compound. The invention also discloses a preparation method of the compound and the kit. The kit can cooperate with a glucometer to detect different HBV types. During detection, a target gene is first hybridized, then the report probe is hybridized, and finally a substrate is introduced to cooperate with a glucometer detection signal to make judgment. The kit disclosed in the invention has the advantages of low cost, easy operation, no need of foreign aid equipment, convenient use and carry, and accurate detection result.
Owner:HUNAN UNIV
Hepatitis B virus typing and resistance assay
The present invention provides methods, kits, and oligonucleotides for detecting and analyzing the nucleotide sequence of a reverse transcriptase (RT) region of the polymerase (Pol) gene of Hepatitis B Virus (HBV). In certain embodiments, a target RT region is amplified and subjected to DNA sequencing. The sequence obtained is compared to one or more DNA sequences characteristic of an HBV genotype or serotype, and / or one or more DNA sequences characteristic of an HBV mutation that confers resistance to a drug or vaccine, to determine the HBV genotype or serotype of the amplified product and / or the presence or absence of one or more DNA sequences characteristic of an HBV mutation that confers resistance to a drug or vaccine.
Owner:ABBOTT MOLECULAR INC
HepG2-based stable-expression HBV (hepatitis B virus) wild strain cell line and preparation method thereof
InactiveCN102816737AConvenient researchExpression level is stableMicrobiological testing/measurementMicroorganism based processesAntigenWild type
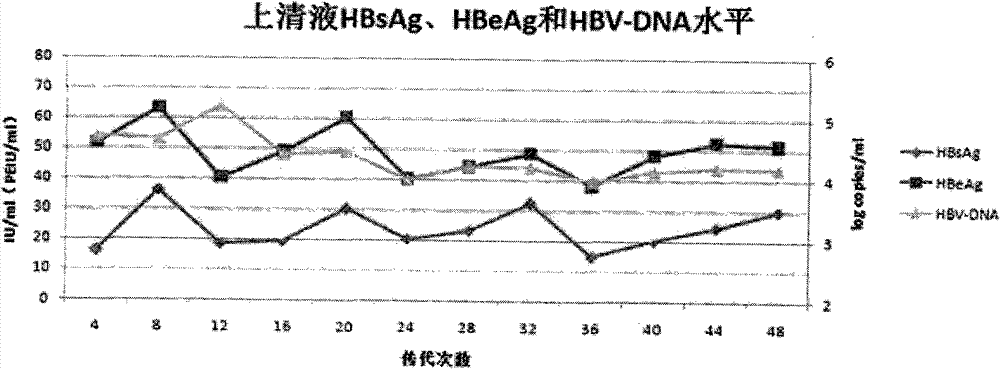
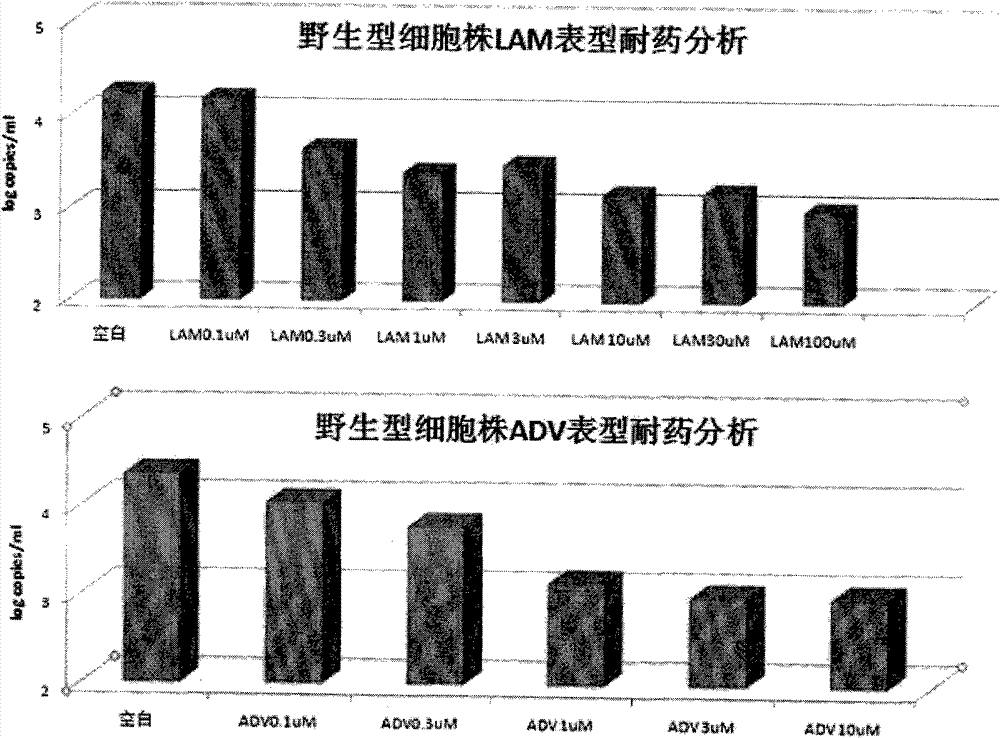
The invention belongs to the field of microorganism animal cell lines, and relates to a HepG2-based stable-expression HBV (hepatitis B virus) wild strain cell line, in particular to a high-expression wild HBV virus liver cancer cell line HepG2.HSWT and a preparation method thereof. The high-expression wild HBV virus liver cancer cell line HepG2.HSWT is obtained by detecting HBV reproduction in cells, specific antigen expression and generation of virus particles through hygromycin screening expression after HepG2 cell transfection of HBV eukaryotic expression plasmid pREP-HBV-WT. The cell line is evidently higher than HepG2.HSWT in expression level of HBsAg and HBeAg and is stable in expression level and less susceptible to factors of culturing environments and the like. The integrated HBV genotype is B or C genotype, cells are sensitive to LAM (lamivudine) and ADV (adenovirus), and HBV intra-cell reproduction level is gradually decreased along with increasing of LAM and ADV concentration. The HepG2-based stable-expression HBV wild strain cell line can be used for researches on Chinese people HBV morbidity or drug resistance mechanisms and screening anti-HBV drugs.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Hepatitis B variants with reduced sensitivity to therapeutic compounds, their detection and uses thereof
ActiveUS20090253123A1Reduce sensitivityReduced responsePeptide/protein ingredientsMicrobiological testing/measurementLong term responseHbv genotype
The present invention is a diagnostic kit and materials for: 1) the prediction of the long-term response of a chronic hepatitis B virus (HBV) carrier to treatment with nucleoside / nucleotide analogue, or their combination; 2) the detection of HBV variants that exhibit reduced reactivity to antibody detection; 3) the detection of HBV variants in the precore / core region that negatively affect the course of liver disease; 4) the identification of the HBV genotype.
Owner:VERSITECH LTD
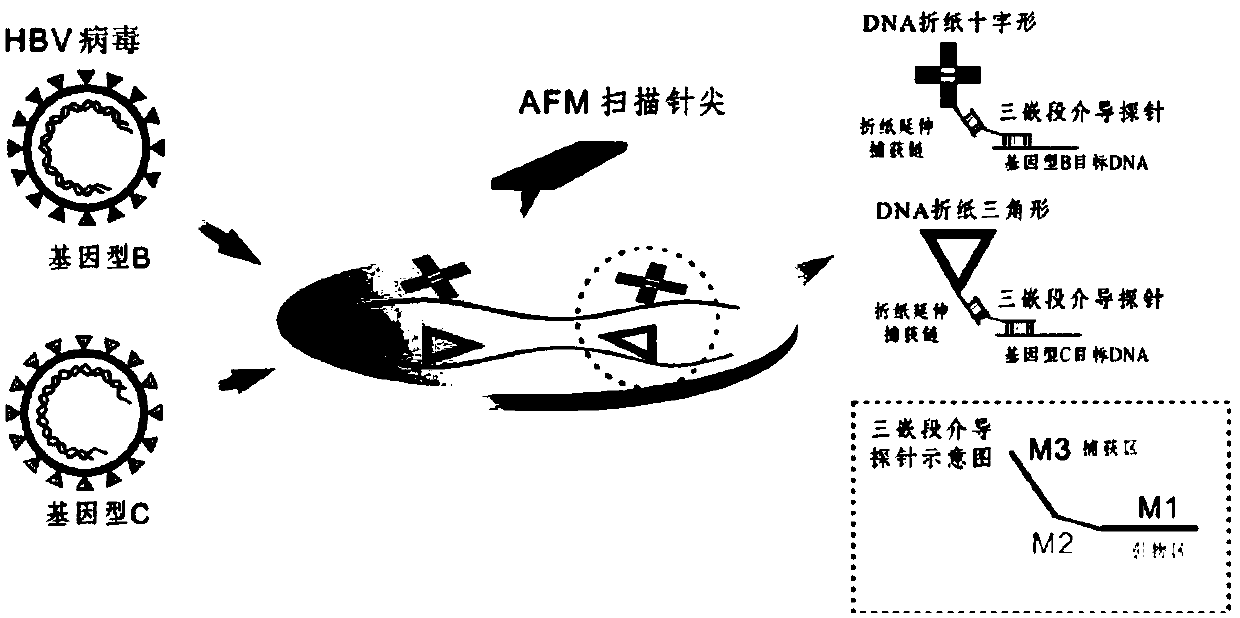
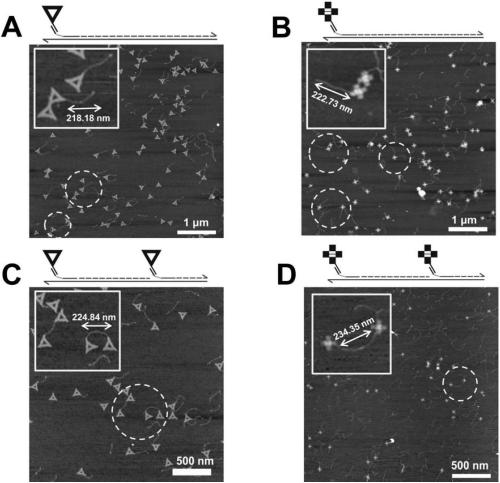
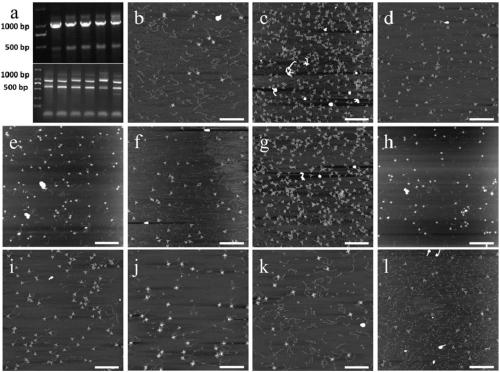
Method for detecting hepatitis b virus subtype based on DNA paper-folding mark
PendingCN110079633AReduce sensitivityRealize typing detectionMicrobiological testing/measurementA-DNAHepatitis B virus
The invention discloses a method for detecting a hepatitis b virus subtype based on a DNA paper-folding mark. A kit provided by the invention includes multiple sets of biological products; each set ofbiological products includes one or multiple sets of reagents; each set of biological products is corresponding to one virus genotype and a DNA folding paper of one shape; each set of reagents is composed of a DNA folding paper with an extending area and a mediate probe corresponding to the DNA folding paper; the extending area of the DNA folding paper in each set of reagents is a free area in size of 54-1119nt extending after 5 Ts extending from one short chain at each endpoint of the DNA folding paper. The invention develops a set of self-assembling method-based DNA folding paper nanometerstructure which is used as a specific mark. A specifically designed probe is used for capturing and marking a clinic hepatitis B virus sample DNA and finally recognizing a corresponding HBV genotype from atomic force microscope (AFM) scanning.
Owner:SHANGHAI JIAO TONG UNIV
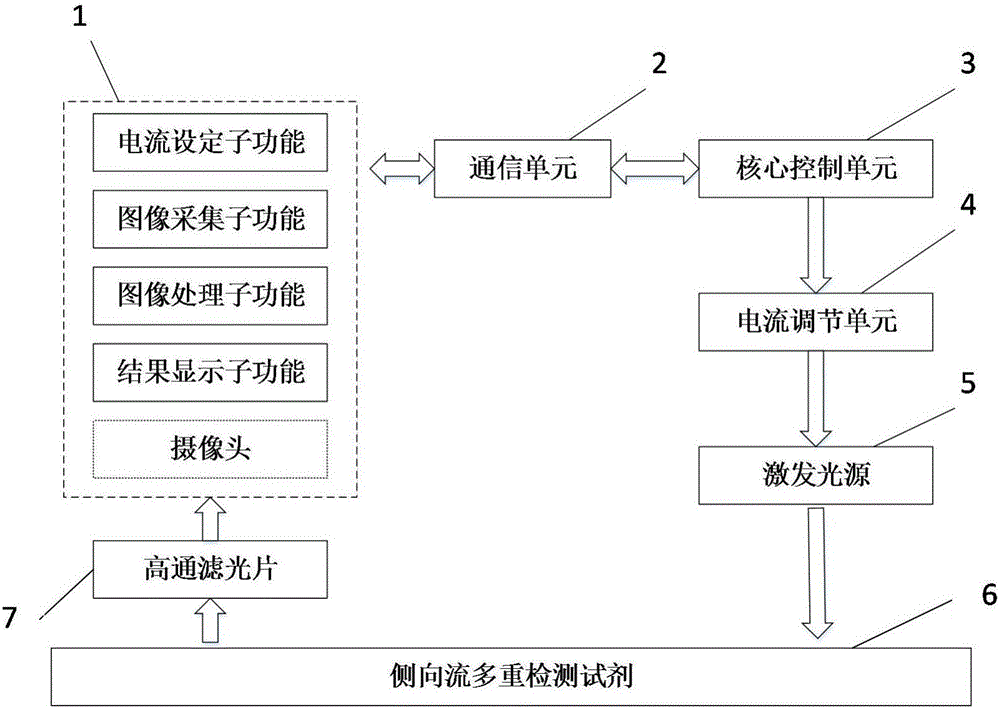
Intelligent equipment based HBV genotyping lateral flow multiplex reagent detection device
InactiveCN106244439ASimple structureReduce volumeBioreactor/fermenter combinationsBiological substance pretreatmentsLuminous intensityFluorescence
The invention relates to an intelligent equipment based HBV genotyping lateral flow multiplex reagent detection device. The device includes intelligent equipment, a communication unit, a core control unit, a current regulation unit, an excitation light source and a high pass optical filter. The intelligent equipment communicates with the core control unit through a bluetooth communication way; the excitation light source is used for exciting the fluorescent substance on a lateral flow multiplex detection reagent, and the exciting light intensity is realized by setting the current regulation unit by the core control unit; and the intelligent equipment shoots the fluorescence image of the lateral flow multiplex detection reagent after passing through the high pass optical filter, image processing is carried out according to a built-in algorithm, the genotype of a to-be-detected sample on the lateral flow multiplex detection reagent is judged, and finally the detection result is displayed. The intelligent equipment is adopted as the system software and image analysis carrier, the structure of the HBV genotyping device is simplified, the detection device has the advantages of small volume, quick detection, simple operation, low cost, and easy promotion, and the detection result can be uploaded to various medical institutions through a wireless network of the intelligent equipment.
Owner:BEIJING UNIV OF CHEM TECH
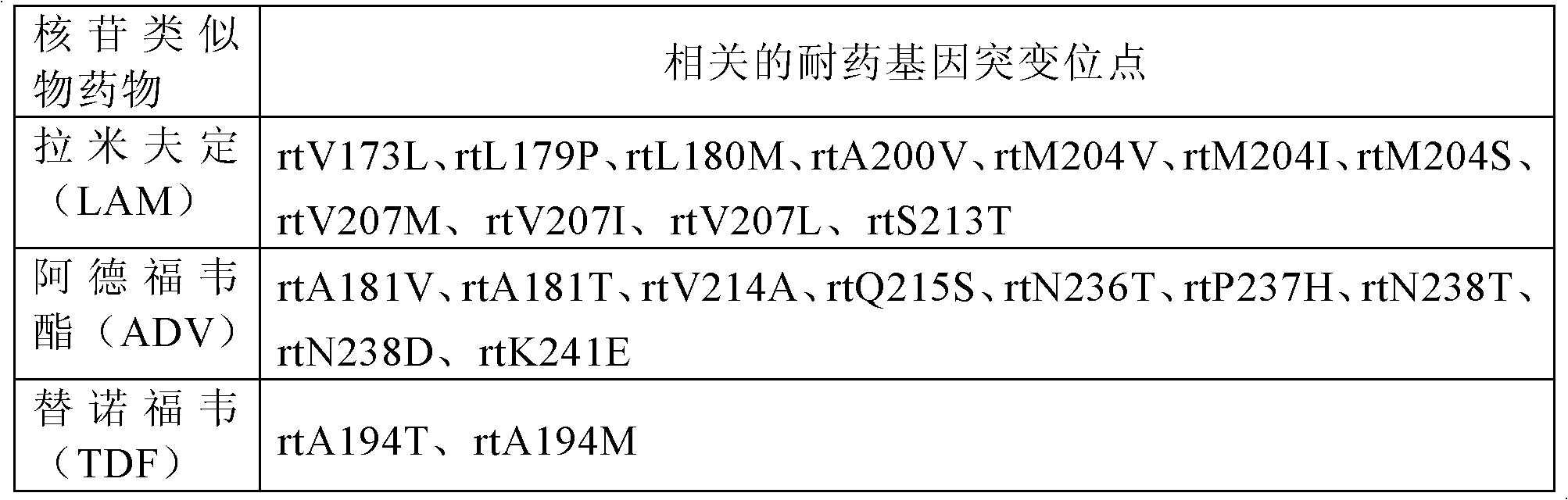
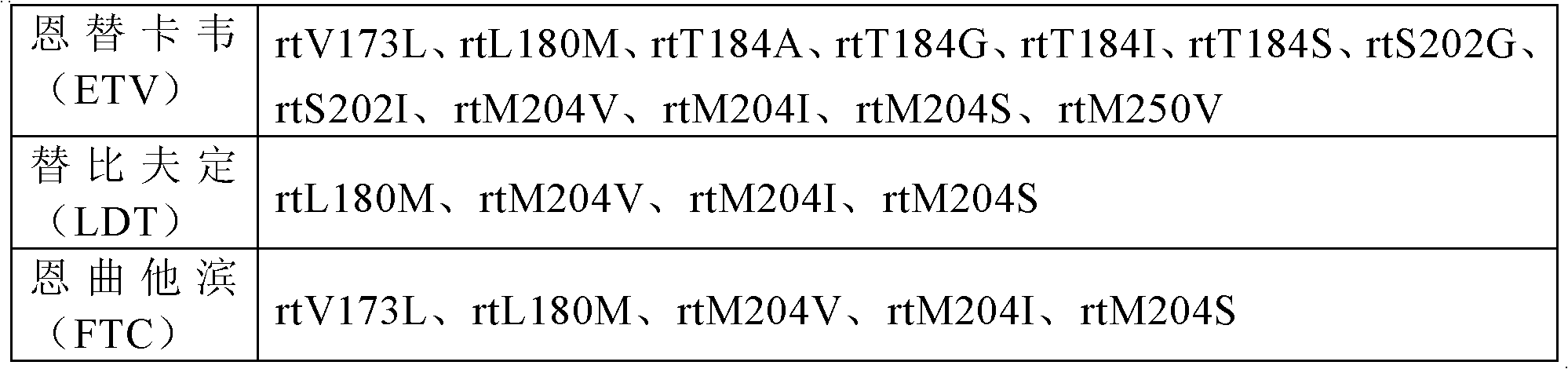
PCR (polymerase chain reaction) primer system for detection of HBV (hepatitis b virus) nucleoside analog drug-resistant mutation sites, method and application
ActiveCN107868848AImprove effectivenessGood repeatabilityMicrobiological testing/measurementHbv genotypeTherapeutic effect
The invention discloses a PCR (polymerase chain reaction) primer system for the detection of HBV (hepatitis b virus) nucleoside analog drug-resistant mutation sites, a method and application. The PCRprimer system comprises a first amplification primer pair, wherein the sequences of an upstream primer and a downstream primer in the first amplification primer pair are respectively as shown in SEQ ID NO:1 and SEQ ID NO:2. The PCR primer system, method and related application can be used for detecting HBV genotype, particularly nucleoside analog drug-resistant mutation sites, thereby being beneficial to the judgment of HBV prognosis and treatment effect, offering help, such as auxiliary clinical medicine, to formulate a personalized antiviral treatment schedules for chronic hepatitis B patients, and ensuring that clinical medicine is more reasonable and more effective. The PCR primer system is high in amplification effectiveness which can reach greater than 97 percent, good in reproducibility, especially low in the minimum detectability, and beneficial to the detection of HBV nucleic acid concentration samples.
Owner:黑龙江金域医学检验实验室有限公司
Guide RNA targets for the treatment of hepatitis B virus infection
ActiveCN104711257BInhibition of replicationGood curative effectGenetic material ingredientsAntiviralsHbv genotypeHepatitis B virus
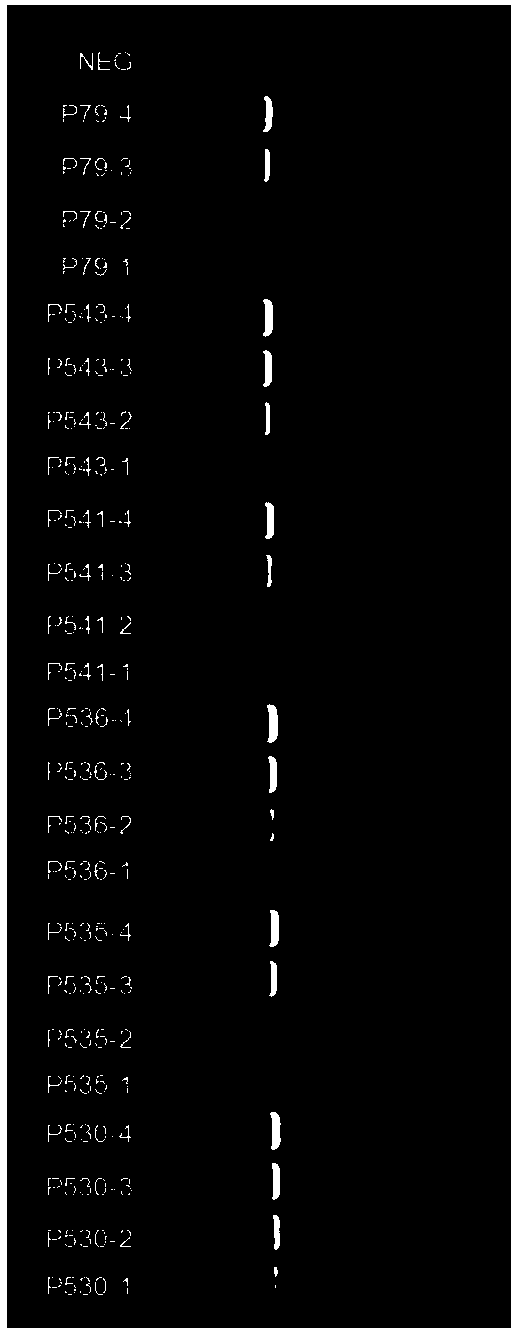
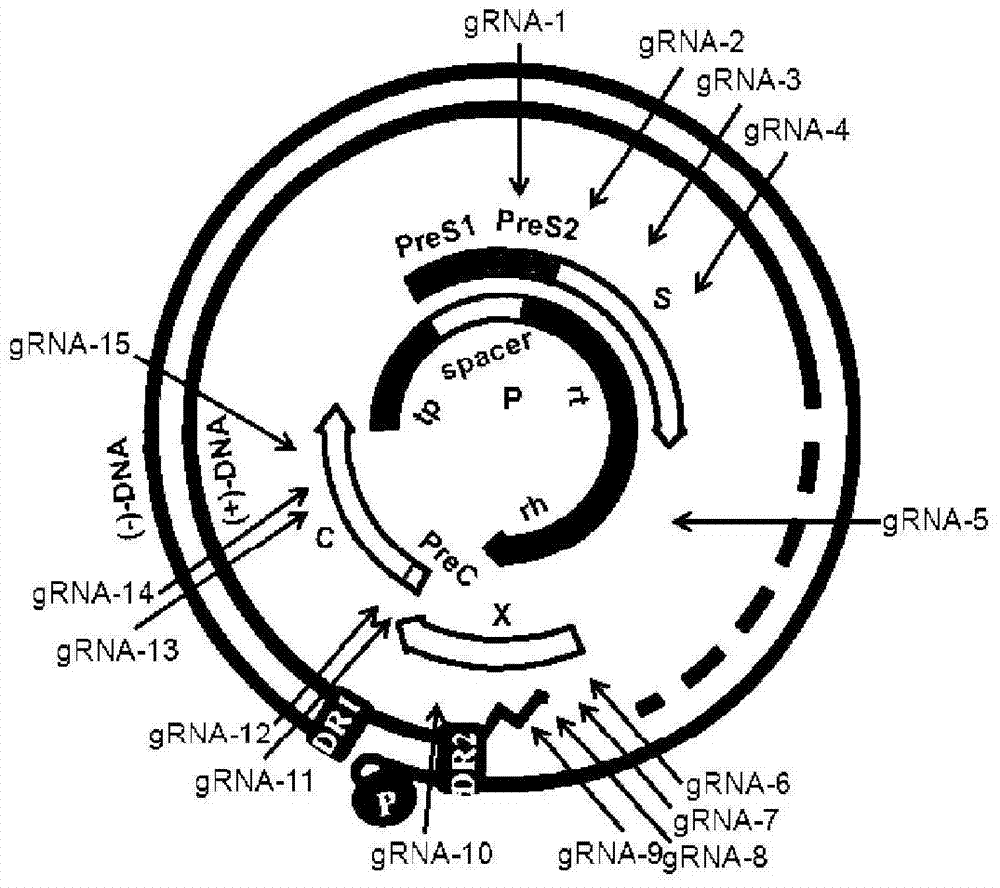
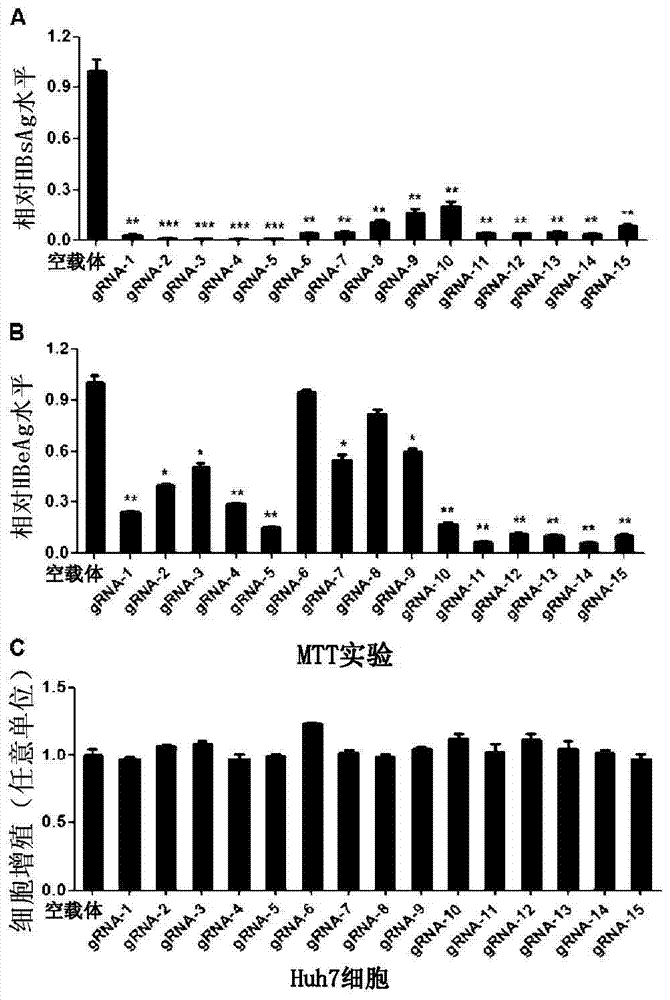
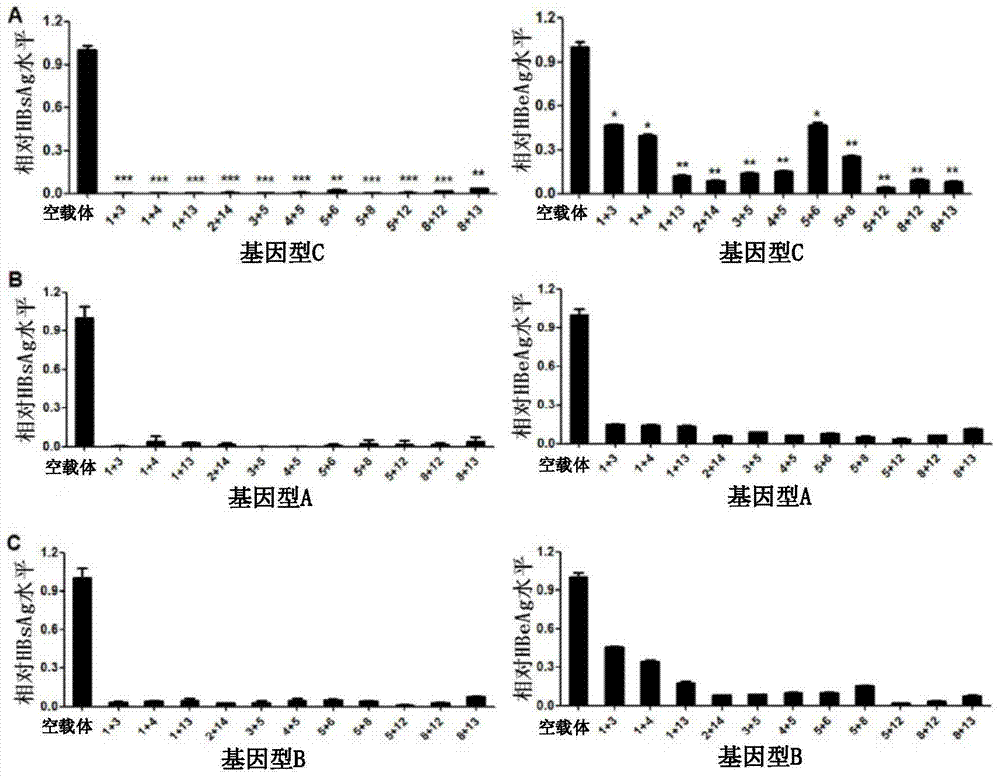
The invention provides 15 guide RNA (short guide RNA, gRNA) targets for the treatment of hepatitis B virus (Hepatitis B virus, HBV) infection, as well as the medicine for preparing the treatment for HBV infection. The invention efficiently destroys multi-genotype HBV covalently closed circular DNA (Covalently closed circular, cccDNA) through the CRIPSR / Cas system, thereby inhibiting HBV replication. These gRNA targets cover multiple regulatory and coding regions of HBV.
Owner:鲁凤民 +2
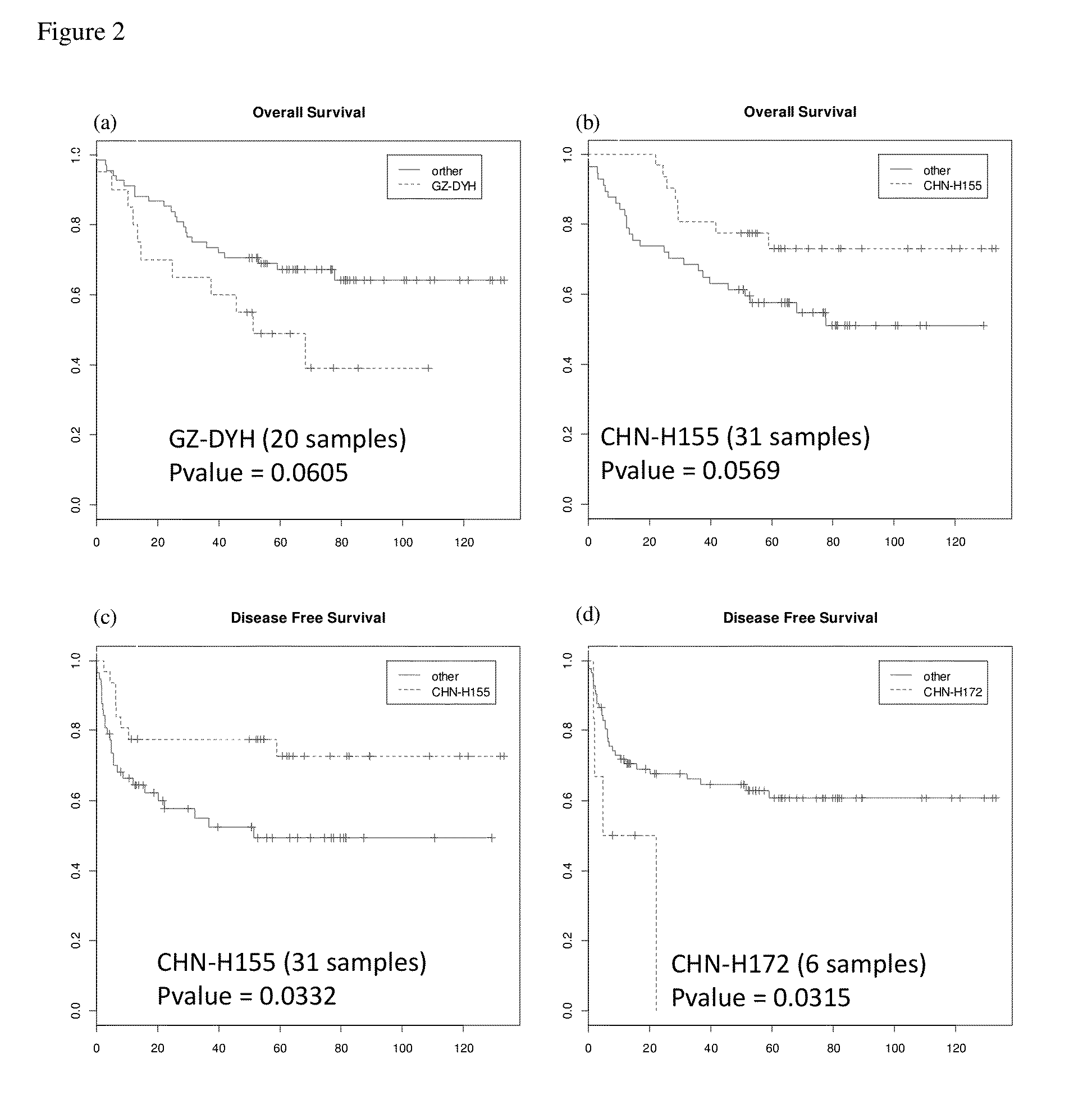
Effect of HBV on clinical outcome of hepatocellular carcinoma cancer patients
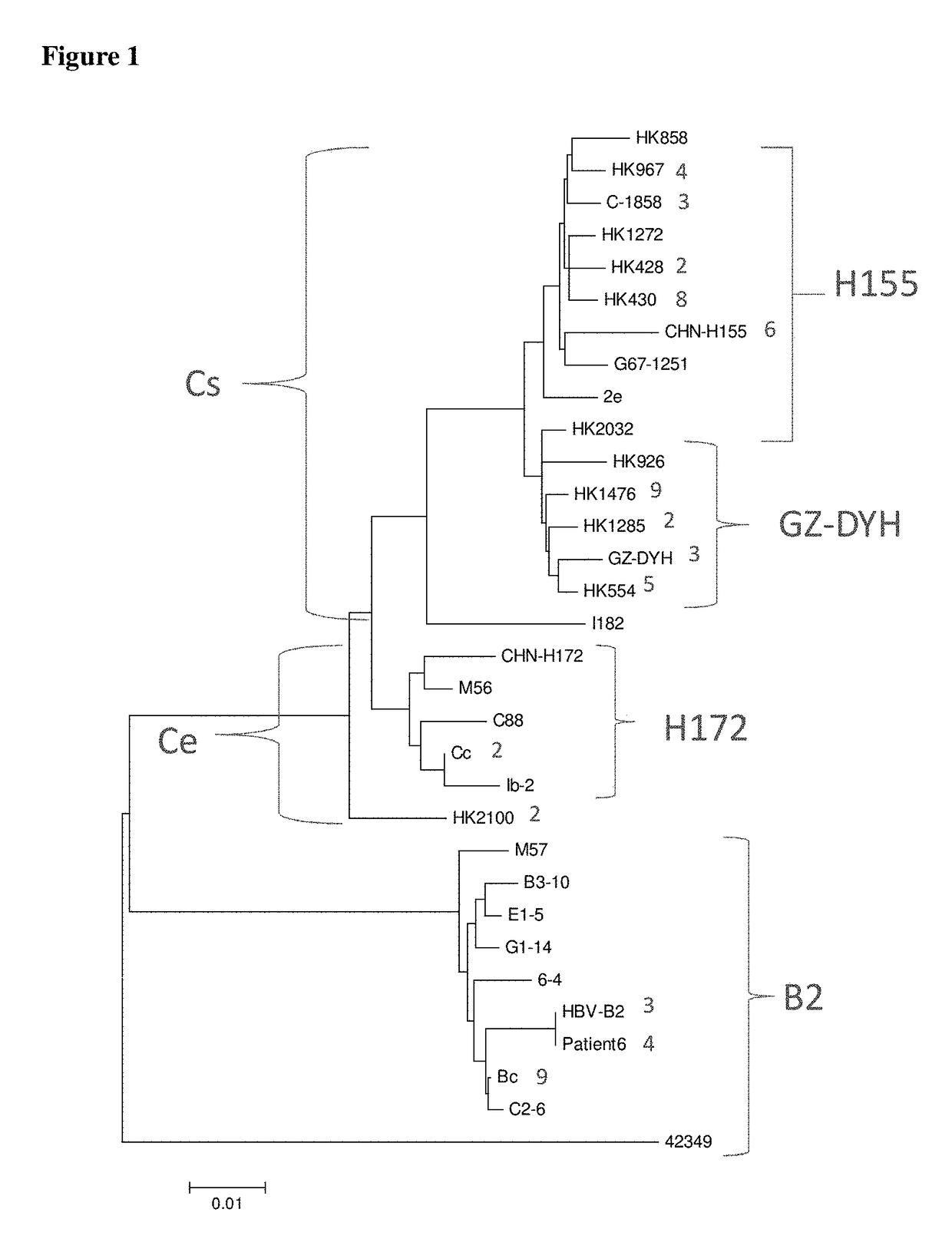
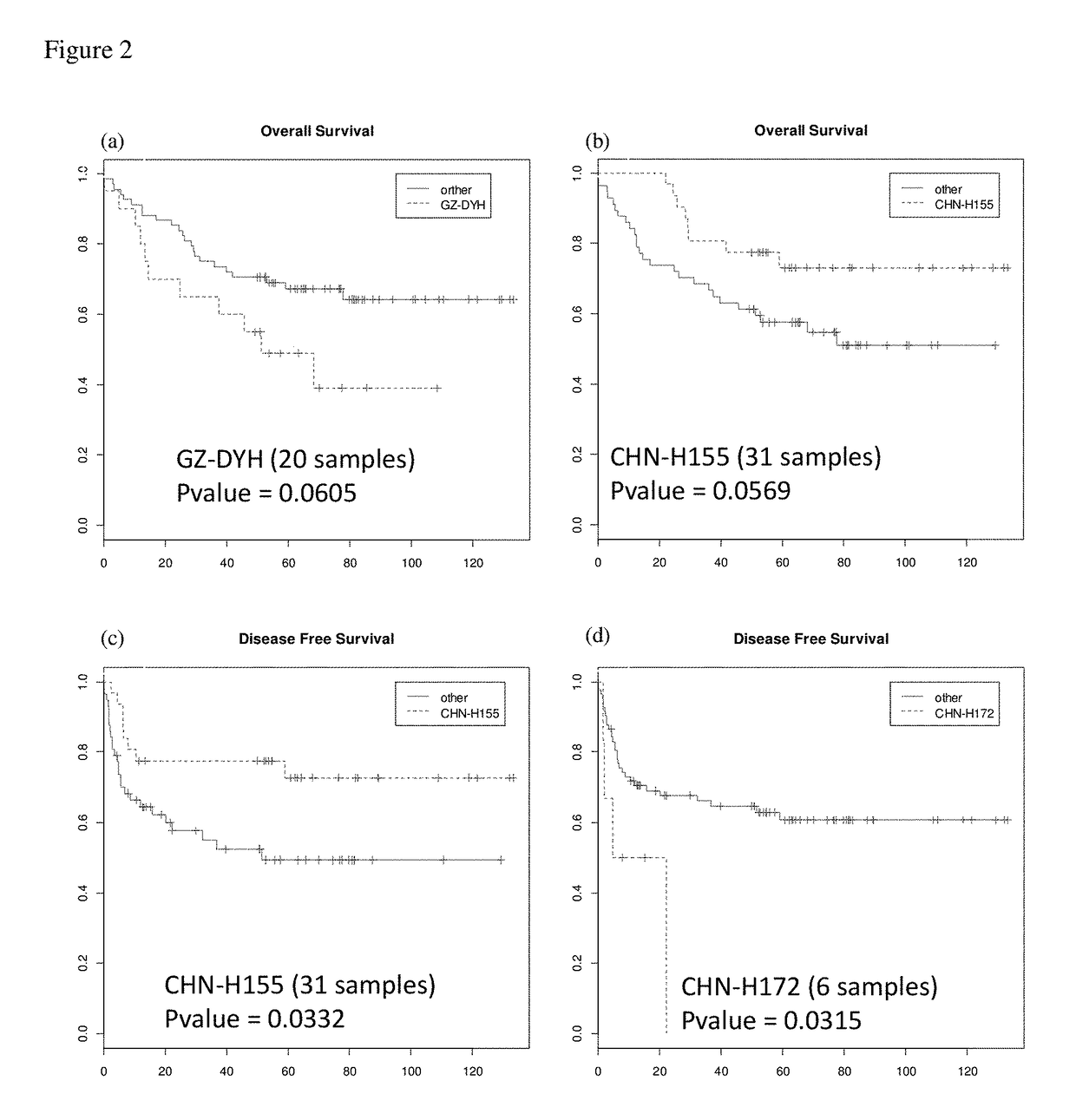
A method for predicting prognosis (clinical outcome) of hepatocellular carcinoma patients based on the detection of a Hepatitis B virus, determination of the HBV genotype, identification of the strain of the HBV genotype and its integration into the genome, in biological samples of such patients is provided.
Owner:AGENCY FOR SCI TECH & RES
Method and kit for detecting HBV genotype and/or X-region mutation, CDS standard sequence of HBx, primer and application
PendingCN111621607AFunction increaseSimple and fast operationMicrobiological testing/measurementMicroorganism based processesHBxHbv genotype
The invention discloses a method and kit for detecting an HBV genotype and / or X-region mutation, a CDS standard sequence of HBx, a primer and application. The method comprises the following steps: selecting CDS of middle HBx of a sequence and CDS standard sequences of 10 kinds of HBx for homology analysis and comparison to obtain a result of the HBV genotypes and / or X-region mutation to be detected; further, judging whether the HBV genotype and / or X-region mutation is a single genotype or a mixed genotype through the peak nesting condition in a sequencing peak graph, and if no nested peak exists or the number of nested peaks is smaller than 10, determining the HBV genotype and / or X-region mutation as the single genotype; and if the number of the nested peaks is greater than or equal to 10,determining the HBV genotype and / or X-region mutation as the mixed genotype. The invention provides a set of convenient and high-accuracy scheme for simultaneously detecting 10 kinds of HBV genotypesand X-region mutation types, and a reference basis can be provided for HBV infected person disease progress risk assessment, antiviral crowd screening, antiviral mode selection and curative effect prediction.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
PCR primer system, method and application for detection of hbv nucleoside analog drug-resistant mutation site
ActiveCN107868848BImprove effectivenessGood repeatabilityMicrobiological testing/measurementHbv genotypeTherapeutic effect
The invention discloses a PCR (polymerase chain reaction) primer system for the detection of HBV (hepatitis b virus) nucleoside analog drug-resistant mutation sites, a method and application. The PCRprimer system comprises a first amplification primer pair, wherein the sequences of an upstream primer and a downstream primer in the first amplification primer pair are respectively as shown in SEQ ID NO:1 and SEQ ID NO:2. The PCR primer system, method and related application can be used for detecting HBV genotype, particularly nucleoside analog drug-resistant mutation sites, thereby being beneficial to the judgment of HBV prognosis and treatment effect, offering help, such as auxiliary clinical medicine, to formulate a personalized antiviral treatment schedules for chronic hepatitis B patients, and ensuring that clinical medicine is more reasonable and more effective. The PCR primer system is high in amplification effectiveness which can reach greater than 97 percent, good in reproducibility, especially low in the minimum detectability, and beneficial to the detection of HBV nucleic acid concentration samples.
Owner:黑龙江金域医学检验实验室有限公司
Hepatitis B variants with reduced sensitivity to therapeutic compounds, their detection and uses thereof
ActiveUS9879330B2Facilitates rapid and easy and accurate detectionImprove visualizationPeptide/protein ingredientsMicrobiological testing/measurementHepatic DiseasesNucleotide
Owner:VERSITECH LTD
A method and kit for detecting hepatitis B virus genotype
InactiveCN101586170BHigh mutation rateAvoid missing detectionMicrobiological testing/measurementMicroorganism based processesType specificOligonucleotide primers
The invention provides a method and a kit for detecting the genotype of hepatitis B virus, the method comprising the following steps: (1) extracting the HBV DNA genome from serum or plasma; (2) designing according to the conserved region of the hepatitis B virus genome Typing primers and probes; (3) Use small molecule labeled oligonucleotide primers to perform PCR reaction to amplify target DNA fragments; (4) Add poly dC tails to oligonucleotide probes, and spot them on the substrate for ultraviolet radiation After linking, bake and fix at 120°C; (5) Use the labeled target DNA amplification product to hybridize with the specific oligonucleotide probe on the substrate; (6) Detect the hybridized conjugates to determine the different genotypes of HBV. The invention designs highly conserved type-specific probes and PCR amplification primers at different base positions, improves detection sensitivity, and can perform HBV genotyping quickly, conveniently and accurately. The invention also provides a test kit for HBV genotyping, which is clinically used for detecting the genotype of hepatitis B virus, can provide reliable basis for clinical treatment and rational drug use, and is worthy of popularization and use.
Owner:CHONGQING MEDICAL UNIVERSITY
Method, oligonucleotide and kit for HBV genotype detection
PendingCN113549707AEasy to detectImprove reliabilityMicrobiological testing/measurementMicroorganism based processesNucleotideHbv genotype
The invention provides a method, oligonucleotide and kit for HBV genotype detection. A fluorescent PCR technology is adopted, not only can determine whether HBV DNA exists in a sample, but also can perform type identification on HBV genotypes A, B, C and D and C / D recombinant types possibly existing in the sample. In addition, HBV genotypes C and D and C / D recombinant types can be accurately distinguished, and targeted selection of therapeutic drugs is facilitated. Besides, non-competitive internal reference detection is added, the detection process of the whole system is monitored, and false negative results can be effectively prevented.
Owner:LEADWAY HK
Probe combination for detection of cancer
ActiveUS11319602B2Highly sensitive, efficient, and reliableDetect presenceSugar derivativesMicrobiological testing/measurementHepatitis B immunizationCancer targeting
A probe combination for detecting cancer includes one or more sets of partial hepatitis B virus (HBV) targeting probes. When sequences of each of the sets of partial HBV targeting probes are aligned, an overall sequence of the aligned set of probes matches a reference sequence of a genome of a HBV genotype or a direct repeat (DR) region on the genome. In the aligned set of probes, each of the probes overlap with one or two adjacent probes by a portion of a length of the probe. The probe combination may further includes one or more sets of hotspot gene targeting probes targeting cancer hotspot genes such as CTNNB1, TERT, and TP53 genes, one or more sets of exogenous gene targeting probes targeting portions of a lambda phage genome, and endogenous gene targeting probes targeting endogenous genes such as GAPDH and GdX genes.
Owner:TCM BIOTECH INT CORP
Effect of hbv on clinical outcome of hepatocellular carcinoma cancer patients
InactiveUS20150275317A1Microbiological testing/measurementLibrary member identificationHbv genotypeIt integration
A method for predicting prognosis (clinical outcome) of hepatocellular carcinoma patients based on the detection of a Hepatitis B virus, determination of the HBV genotype, identification of the strain of the HBV genotype and its integration into the genome, in biological samples of such patients is provided.
Owner:AGENCY FOR SCI TECH & RES
Polymerase chain reaction detection method of hepatitis B virus genotyping
ActiveCN102140537BSimple and fast operationHigh compliance rateMicrobiological testing/measurementFluorescence/phosphorescenceSerum samplesFluorescence
The invention provides a polymerase chain reaction (PCR) detection method of hepatitis B virus (HBV) genotyping. The method comprises the following steps: a primer is designed according to the entire genome sequence of the HBV genotype; in the existence of the primer, DNAs are extracted from a serum sample to perform PCR amplification in a fluorescence PCR meter, the PCR product is analyzed according to the melting curve, the genotype is judged according to the Tm value of the PCR product and the HBV genotyping can be realized. The PCR melting curve method used in the invention is convenient to operate, the detection result of the method has higher coincidence rate with the detection result obtained by adopting a commercial genotyping kit; and the method can be used in the clinical detection of the HBV genotype. On the basis of the PCR technology, the PCR melting curve method which is to judge the HBV genotype according to the Tm value, is provided in the PCR detection method, thus the HBV genotype can be identified conveniently, rapidly and accurately.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com