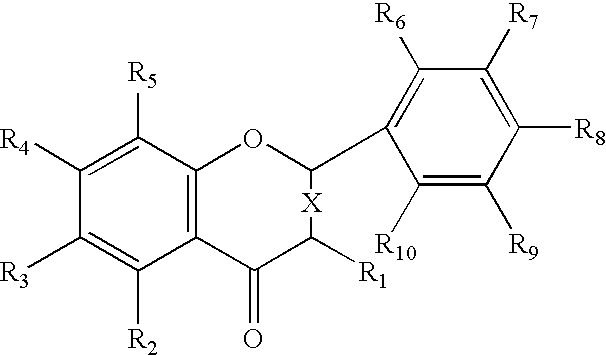
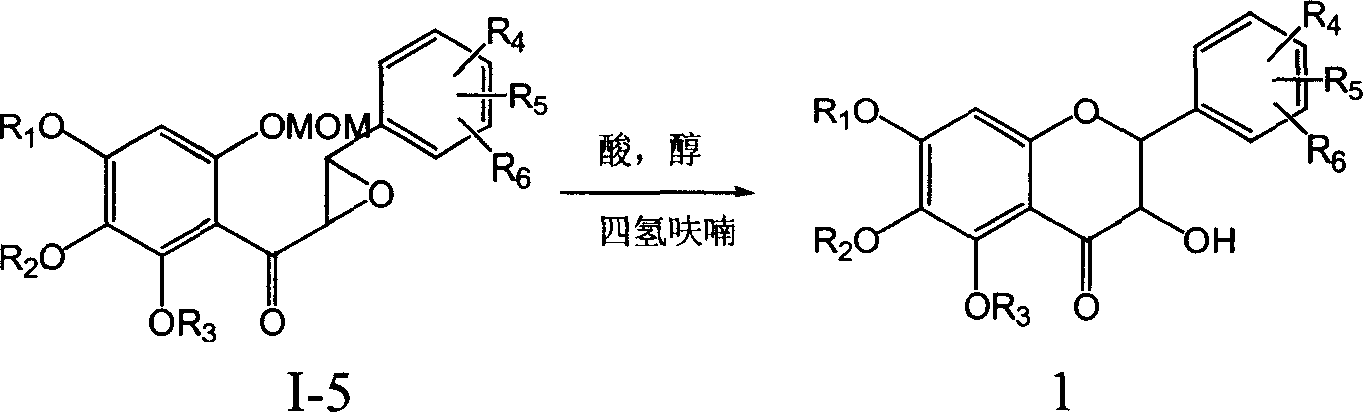
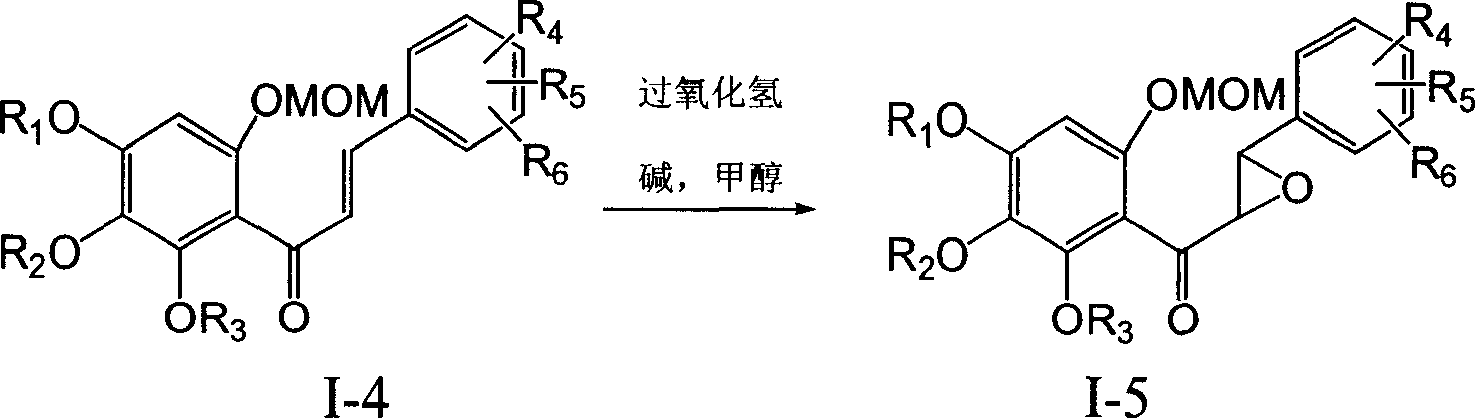
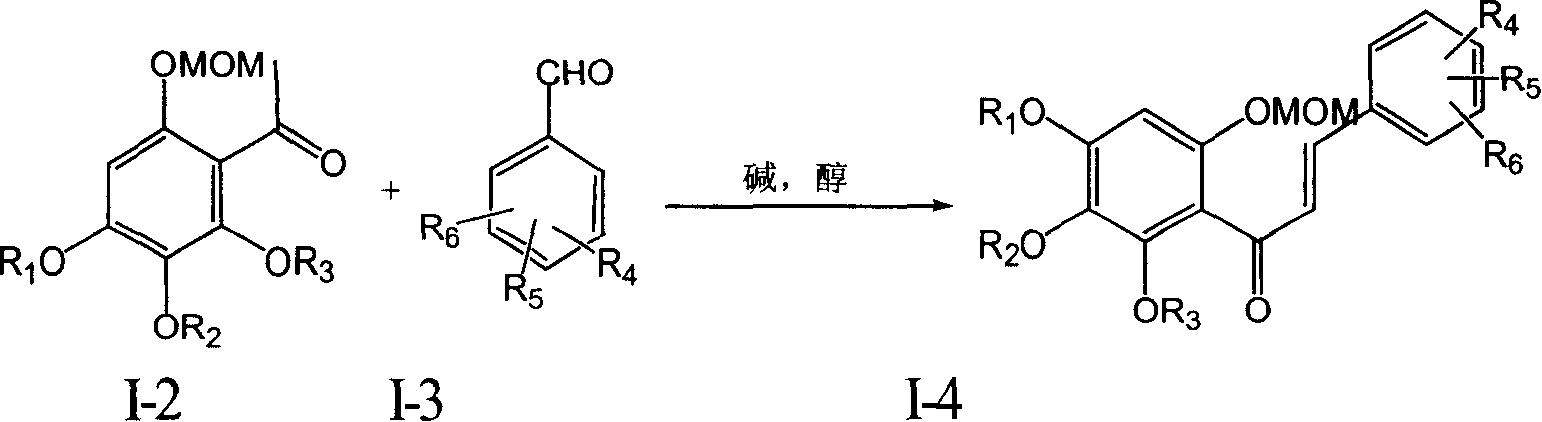
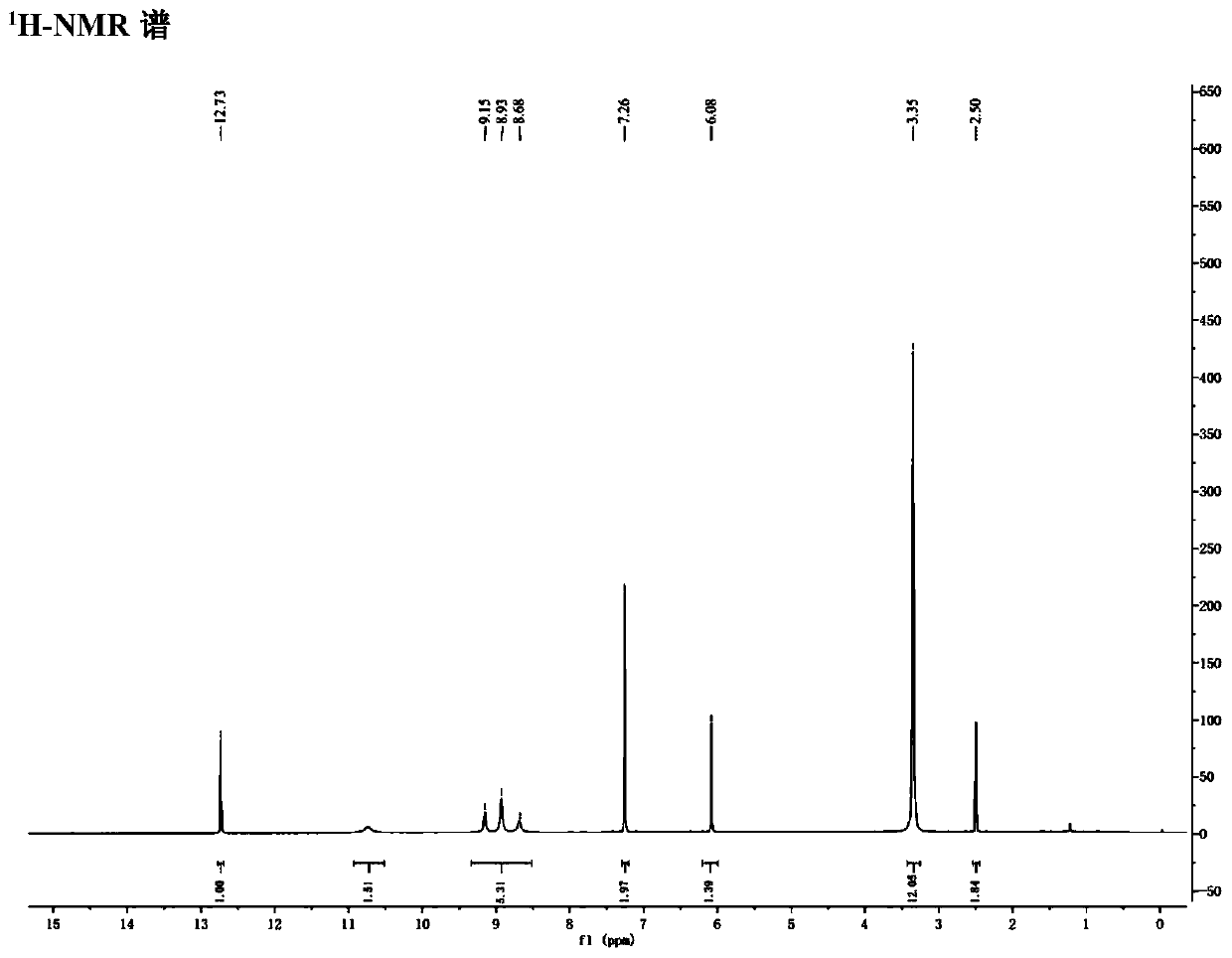
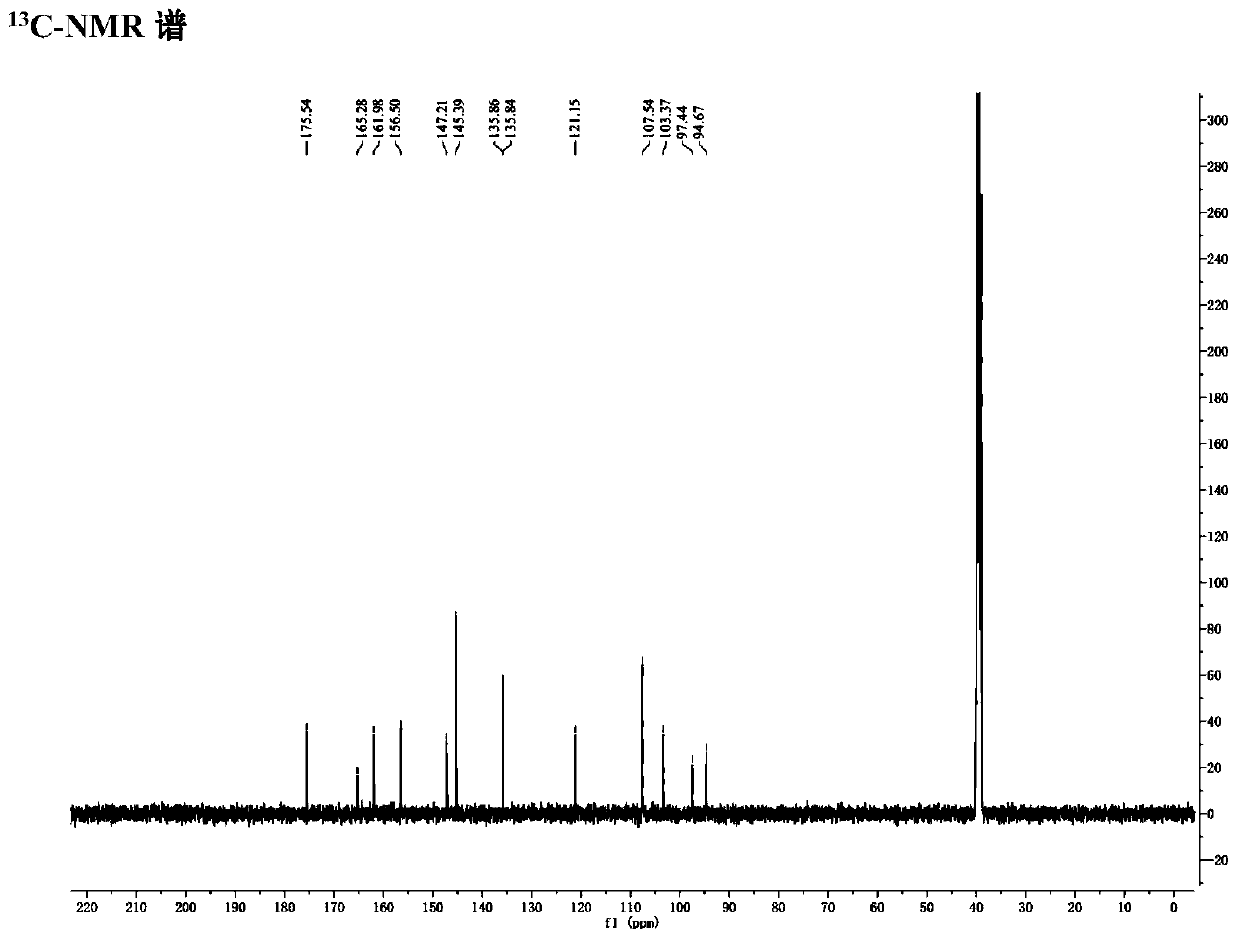
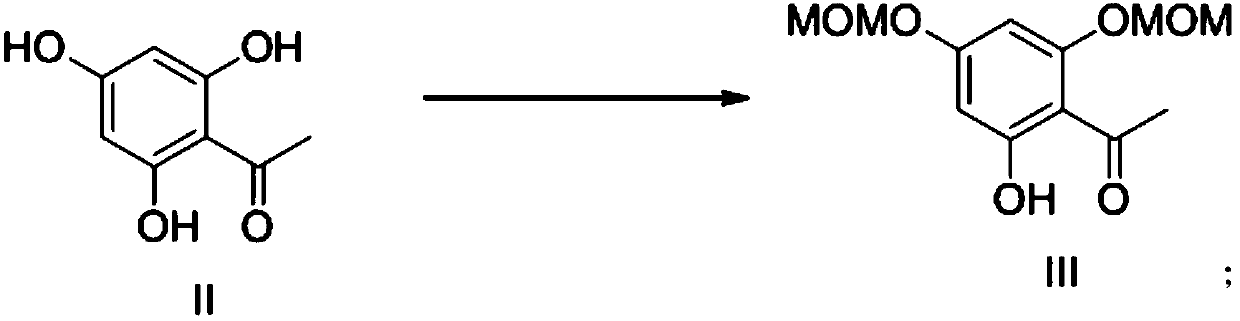
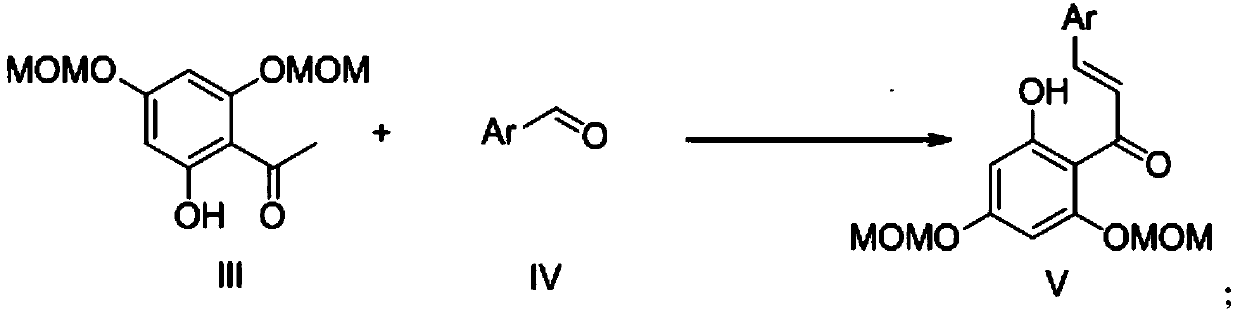
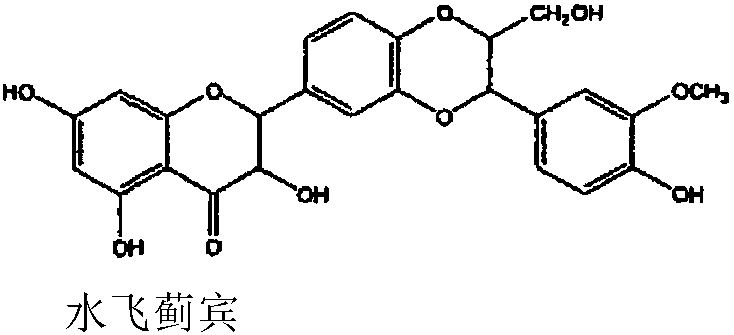
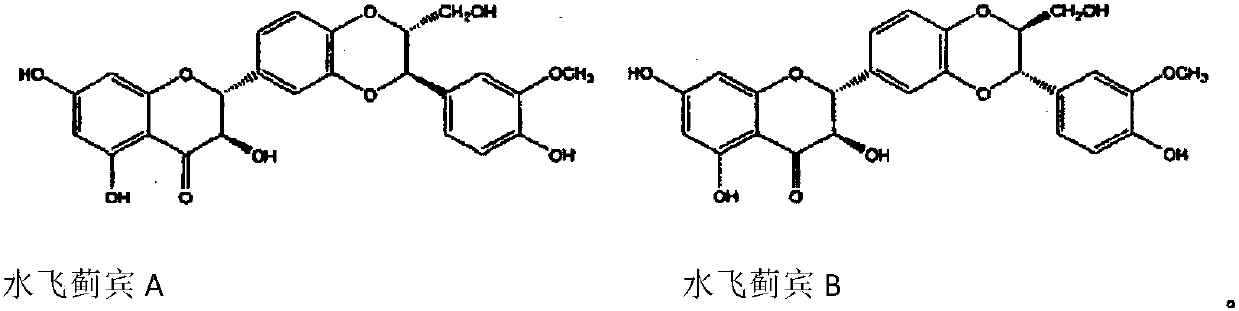
Patents
Literature
34 results about "Flavanonol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
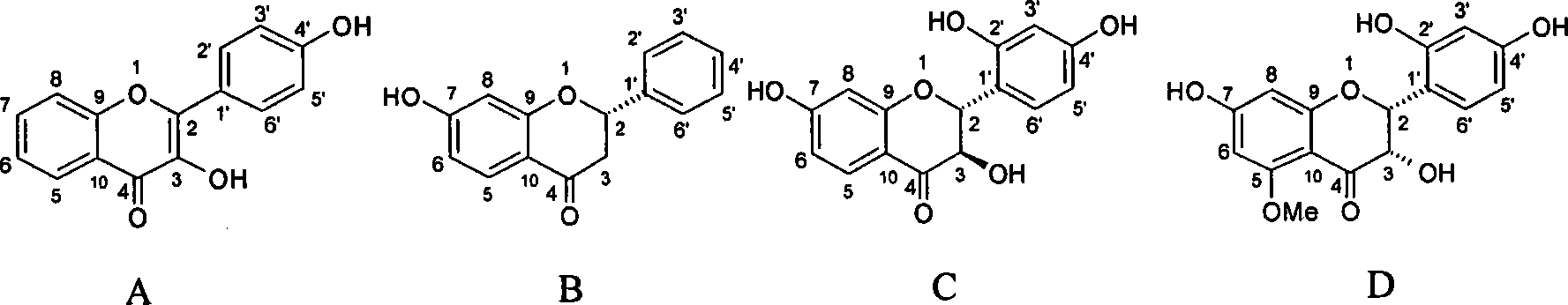
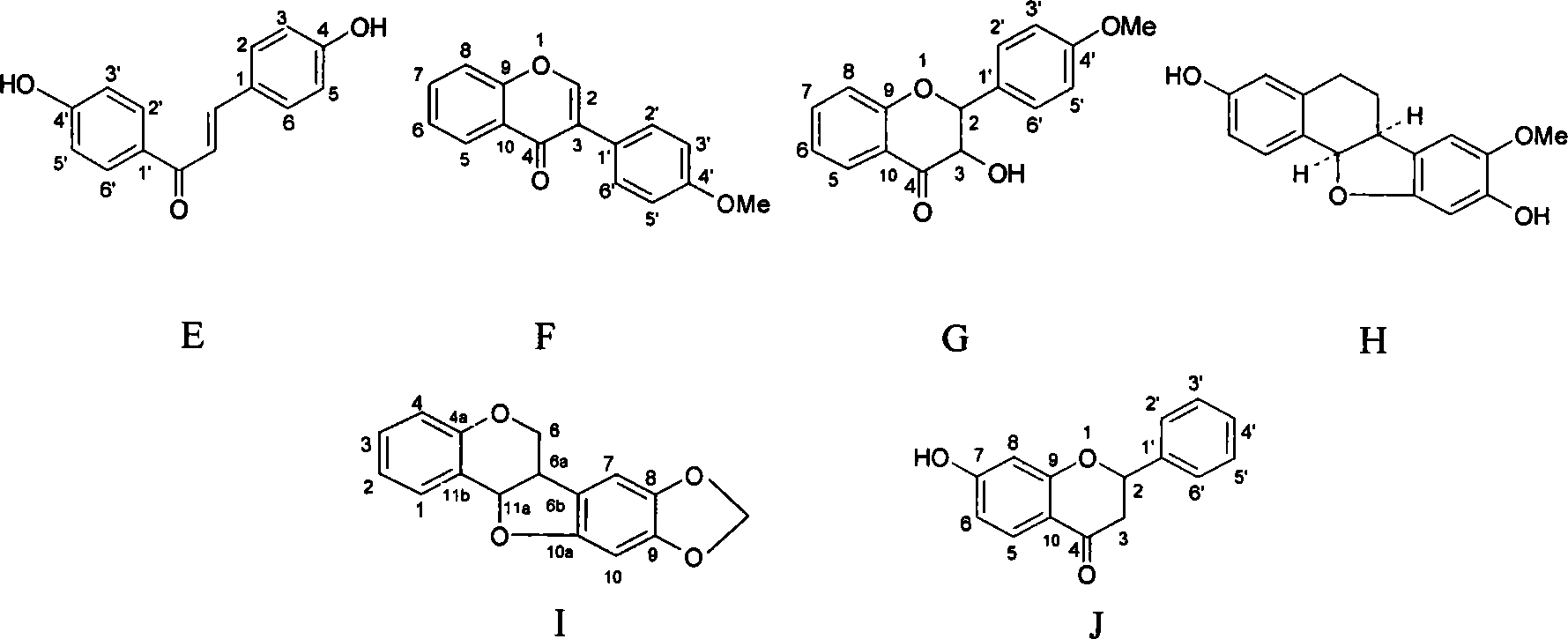
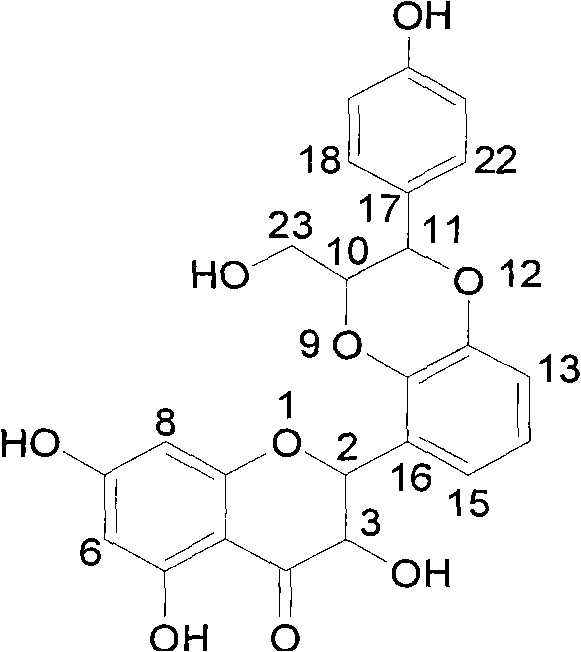
The flavanonols (with two "o"s a.k.a. 3-hydroxyflavanone or 2,3-dihydroflavonol) are a class of flavonoids that use the 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one (IUPAC name) backbone.
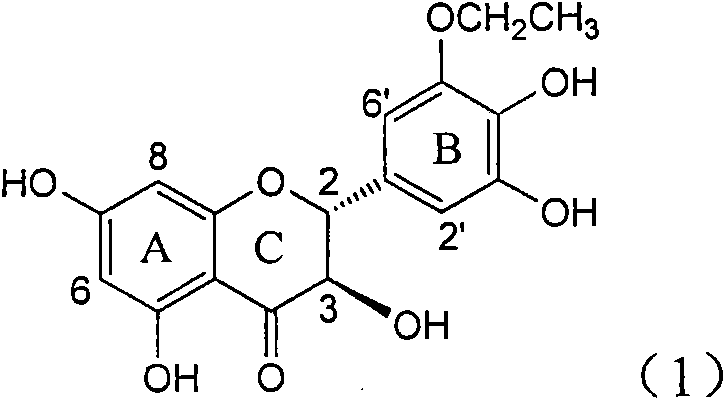
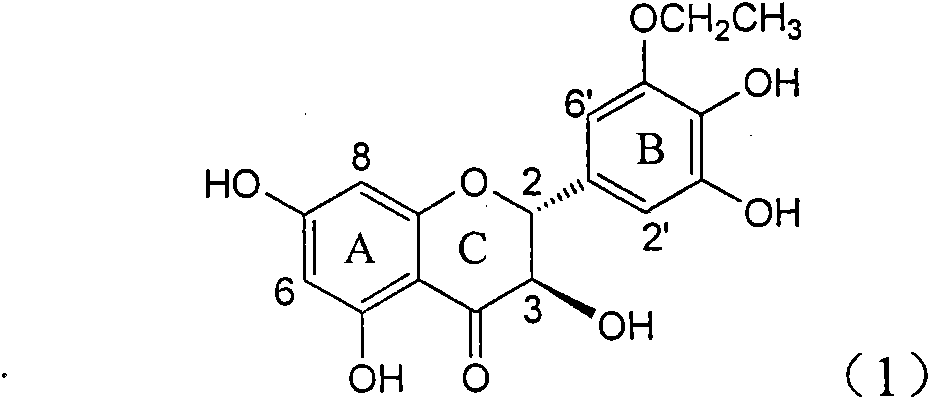
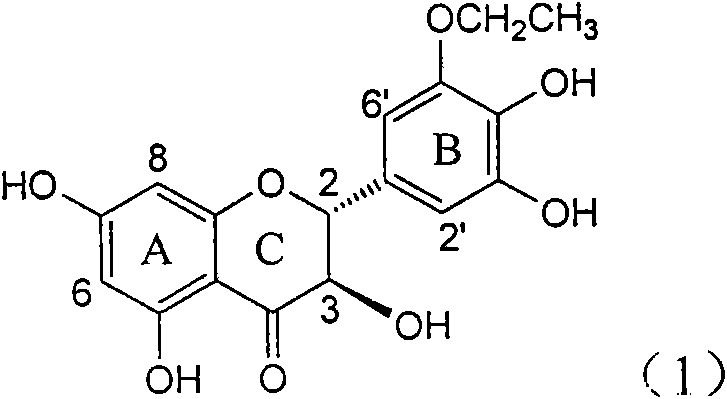
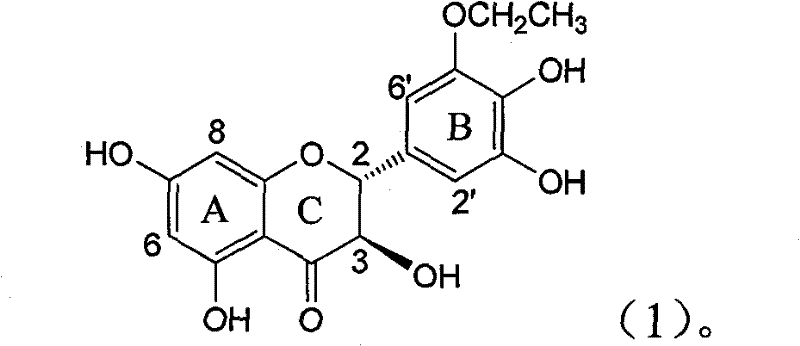
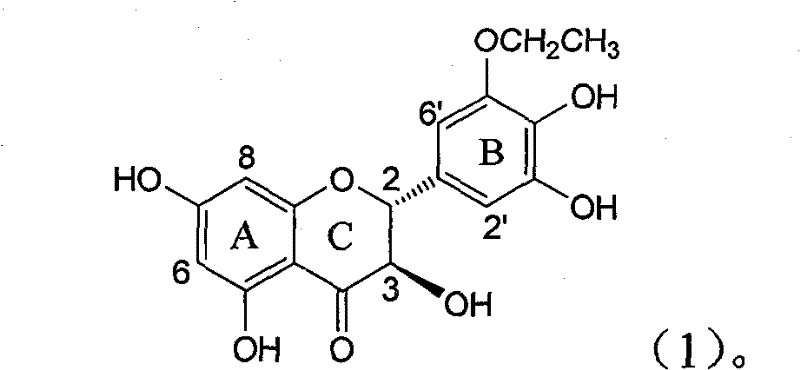
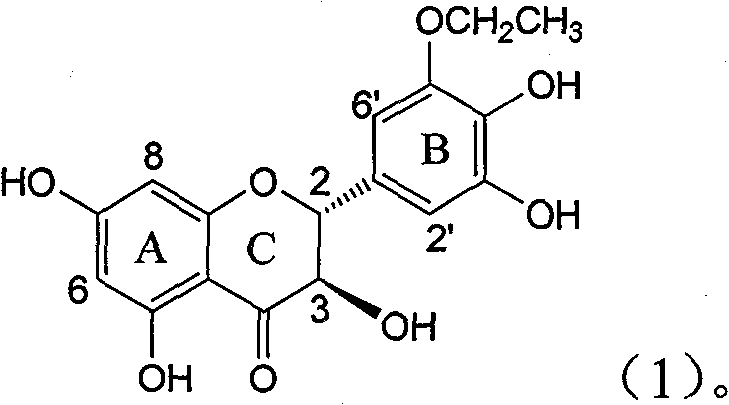
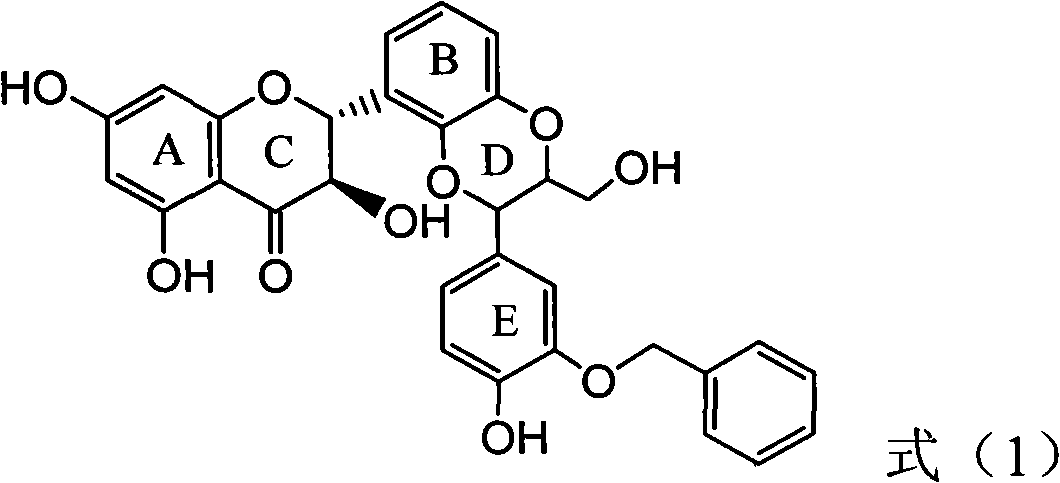
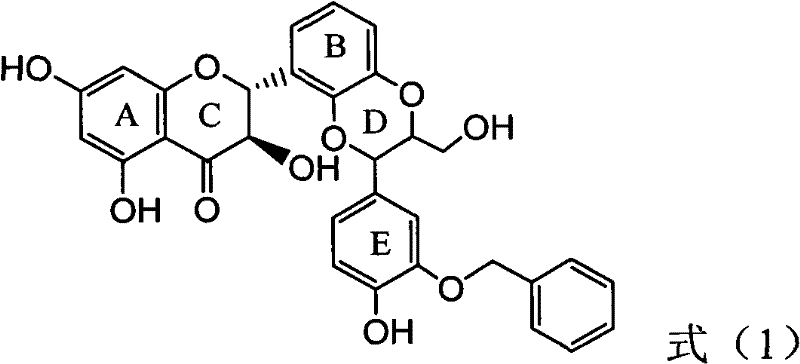
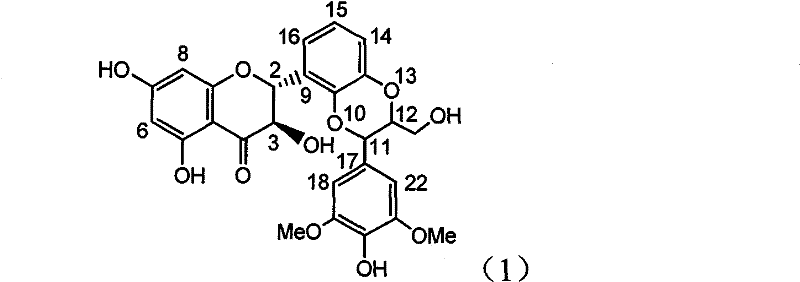
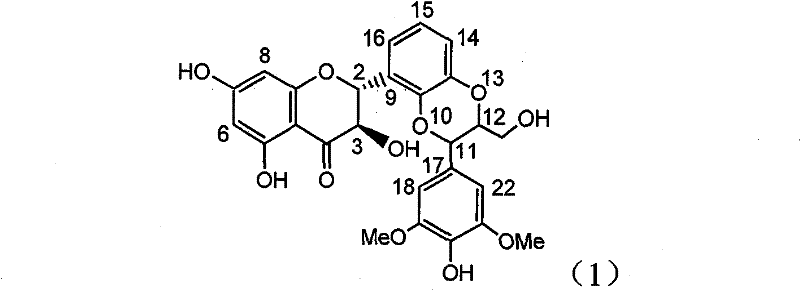
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
InactiveCN101822664AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
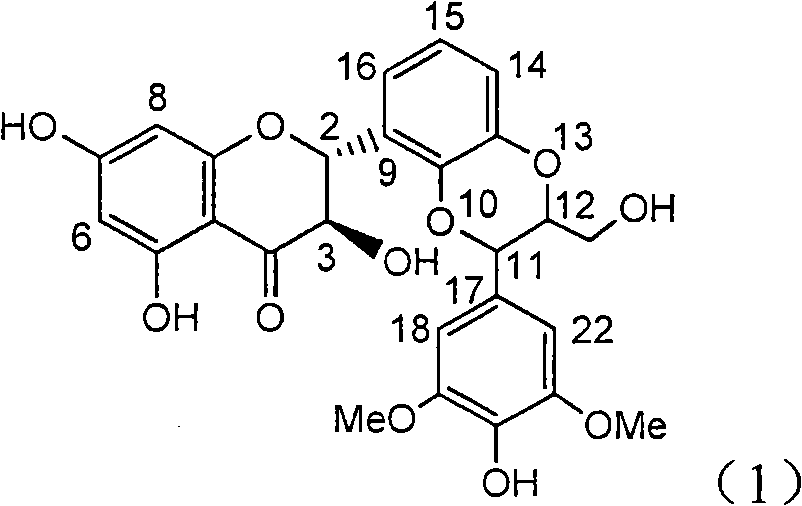
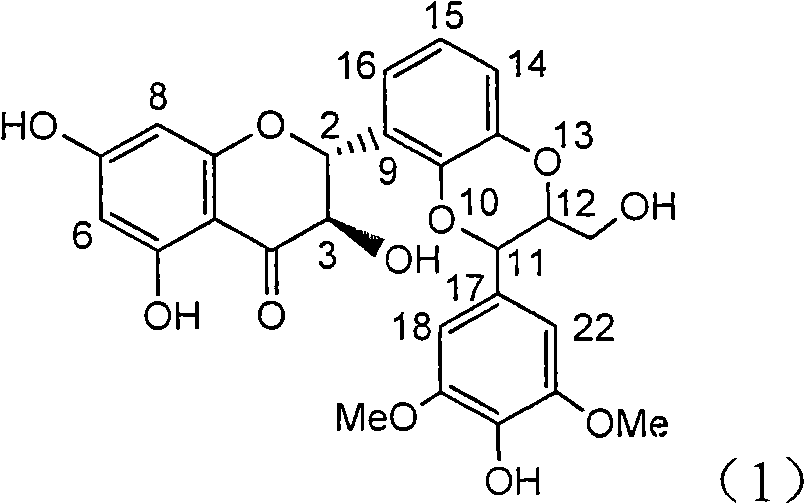
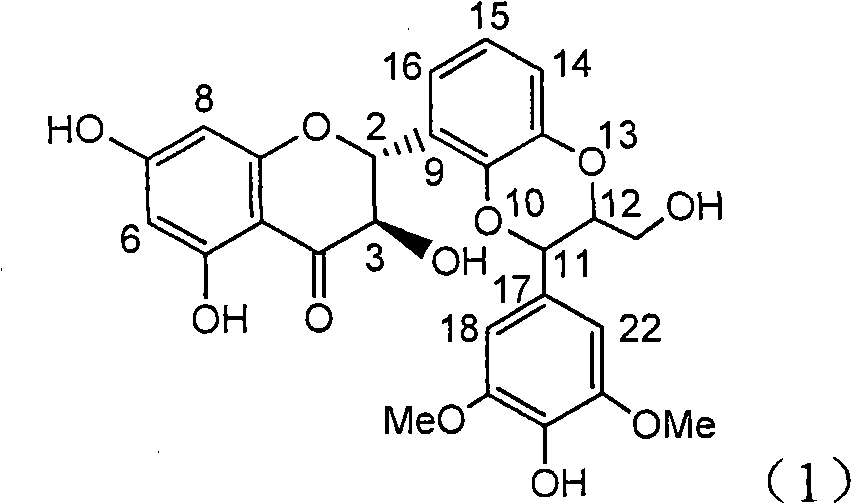
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of 20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
Method for preparing general flavone and total alkaloid of sophora flavescens simultaneously
The invention discloses a method for simultaneously preparing total flavone extract and total alkaloid extract of Radix sophorae flavescentis. Total flavone extract mainly comprises flavanones (flavanonols) such as norkurarinone, kurarinone, and isokurarinon and other flaconoids such as trifolirhizin, xanthohumol, and kuraridin. Total alkaloid extract mainly comprises matrine n-oxide, N-oxysophocarpine, matrine, sophocarpine, sophoridine, and sophoramine. Total flavone extract and total alkaloid extract can be extracted through one or more of methods of solvent-extraction, solvent extraction, macroporous adsorbent resin, extraction by supercritical fluid, column chromatography, and liquid-liquid countercurrent distribution chromatography. Flavonoid content in total flavone extract is 5-100wt%, wherein the content of norkurarinone, xanthohumol, kuraridin, and trifolirhizin occupies 1-100wt%.Alkaloid content in total alkaloid extract is 5-100wt%, wherein the content of matrine n-oxide, N-oxysophocarpine, matrine and sophocarpine occupies 5-100wt%.
Owner:石任兵 +1
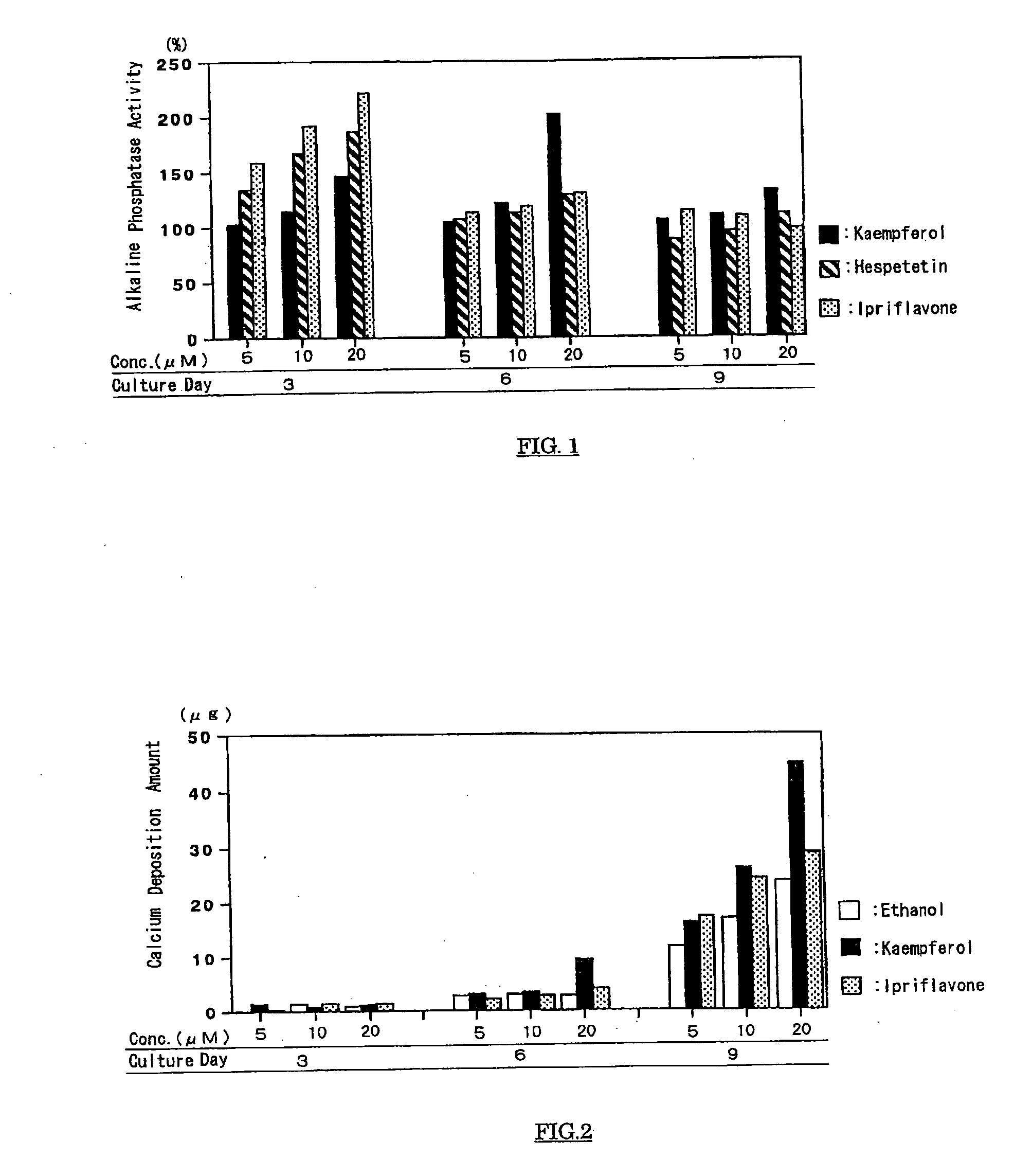
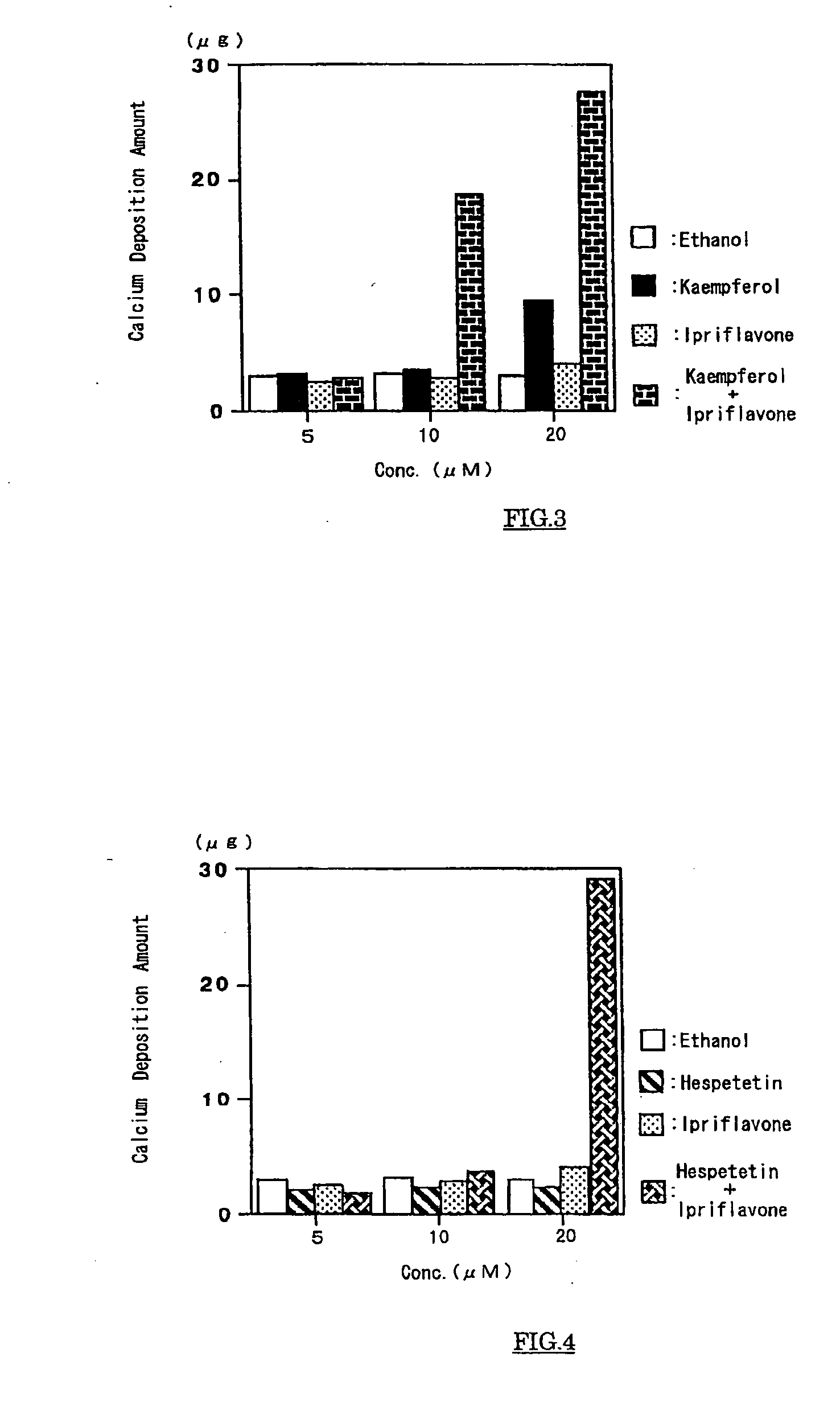
Calcium-containing tissue strengthening agents and use thereof
InactiveUS20050215493A1Increase bone massIncrease depositionCosmetic preparationsBiocideFlavanoneFlavonols
The present invention has an object to provide an agent for strengthening calcium-containing tissues, which can be safely applied; and its use: The present invention solves the object by providing an agent for strengthening calcium-containing tissues, which comprises one or more flavones, flavonols, flavanones, flavanonols, anthocyanidins, flavanols, chalcones, and aurones.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Extraction separation method of a flavone component based on graphene
ActiveUS20160145229A1Increase separation speedIncrease surface areaOrganic chemistryFlavanoneGraphene
The present invention refers to the technical field of flavone component extraction, and provides an extraction separation method of a flavone component based on amination graphene. The flavone components comprise flavones, flavanols, isoflavones, flavanones, flavanonols, flavanones, anthocyanidins, chalcones, and chromones etc. The extraction separation method is adsorption extraction, and amination graphene is taken as a medium of adsorption extraction. The extraction separation method of the flavone components based on amination graphene is superior in separation speed and product purity, low cost and convenient operation.
Owner:SHENZHEN XIHAN MEDICAL & HEALTH ENVIRONMENTAL PROTECTION CO LTD
Flavanonol compounds and their production method and use
InactiveCN1990482AHas a growth effectGrowth inhibitory effectOrganic active ingredientsAntimycoticsImmunologic disordersAbnormal tissue growth
The invention relates to a novel 5, 6, 7- tri-oxygen Dihydroflavonol, shown in formula (1), the preparation method and its application. The compound can be used to prepare drug combination that can prevent hepatitis b, protect liver, treat fungal infection, prevent diseases that generates free radical during disease change including inflammation, autoimmune diseases, radiotherapy sequela, tumor, myocardial ischemia, cardiac hypertrophy, aging, allergic reaction and atherosclerosis. There are various preparations for said drug combination.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Botanical antioxidant compositions and methods of preparation and use thereof
ActiveUS9326932B2Prevent damage to skinBroad protectionCosmetic preparationsToilet preparationsAntioxidantIsoflavones
A botanical antioxidant composition includes a botanical antioxidant extract blend including a first antioxidant botanical extract including at least one hydroxycinnamic acid, and at least one additional antioxidant botanical extracts including at least one antioxidant selected from the group consisting of vitamins, stilbenoids, curcumininoids, tannins, flavones, flavonols, flavan-3-ols, flavanones, anthocyanidins, anthocyanins, isoflavones, flavanonols, proanthocyanidins, dihydroxybenzoic acids, and pyridine alkaloids.
Owner:DERMAFORCE HLDG LLC
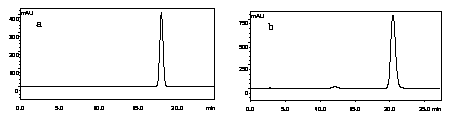
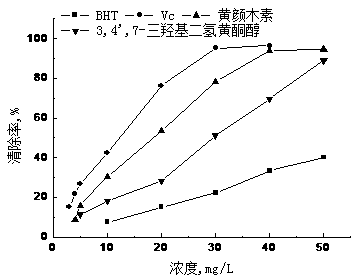
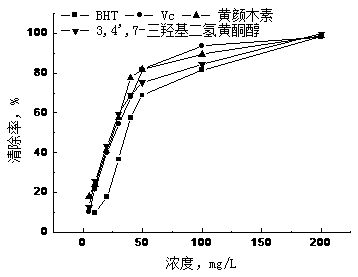
Method for extracting 3,4',7-trihydroxy flavanonol and fustin with antioxidant activity from varnish tree wood meal
ActiveCN105669627AAvoid decompositionHigh extraction rateOrganic chemistryAntinoxious agentsAdditive ingredientSilica gel
The invention relates to a method for extracting 3,4',7-trihydroxy flavanonol and fustin with the antioxidant activity from varnish tree wood meal, and belongs to the field of development and application of plant extracts and chemistry of natural products. The varnish tree wood meal is used as a raw material, extraction, silica gel column chromatography and middle-lowerpressure chromatography are performed to obtain the 95% or above 3,4',7-trihydroxy flavanonol and fustin. The ingredients with the efficient antioxidant activity are obtained from a varnish tree, and the removal rate of DPPH, ABTS and hydroxyl radicals is higher than 95% when the concentration is 50 mg / L or above. The preparation process is rapid and simple, the extracted 3,4',7-trihydroxy flavanonol and fustin are good sources for developing a potential natural antioxidant, and the good commercial prospect is achieved.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Process for preparing diselenide compound
ActiveCN111454240AMild reaction conditionsSmooth responseOrganic chemistryCombinatorial chemistryFlavonols
The invention provides a process for preparing a diselenide compound. The method comprises the following steps: adding a polar solvent into a flavanonol compound; heating the solution to 80 DEG C to 100 DEG C, keeping the temperature for 40-50 minutes, then adding alkali of which the amount of substance is 0.4-1.0 time of that of the flavanonol compound, and carrying out a reaction for 5-60 minutes at the temperature of 80-100 DEG C; then adding selenium dioxide of which the amount of substance is 0.6-1.2 times of that of the flavanonol compound; and carrying out a reaction for 30-150 minutesat the temperature of 80-100 DEG C to obtain the diselenide compound of the dihydroflavonol compound. The method disclosed by the invention is mild in reaction, small in pollution and suitable for large-scale industrial production, and does not need an anhydrous oxygen-free environment.
Owner:SHANGHAI SPARK PHARM CO LTD
Emulsifying cosmetics for eyebrows or eyelashes
ActiveCN103732208BExcellent hair growth effectPrevent drynessCosmetic preparationsHair cosmeticsHydrogen atomIrritation
An emulsified cosmetic for eyebrows or eyelashes with reduced irritation to the eyes and capable of suppressing the occurrence of dryness of the skin around the roots of the eyebrows or eyelashes and of retaining elasticity of the skin is provided. The emulsified cosmetic contains 0.01 to 1% by mass of a flavanonol derivative represented by the following formula (1) (wherein R 1 and R 2 are each independently a hydrogen atom or a methyl group) and 5 to 65% by mass of a volatile oil selected from volatile hydrocarbon oils and volatile silicone oils. The flavanonol derivative is dissolved in the volatile oil.
Owner:KAO CORP
Method for increasing proanthocyanidin content in escherichia coli by cotransformation of brassica juncea gene BAN and DFR
InactiveCN102827849AIncrease proanthocyanidin contentBacteriaMicroorganism based processesBiotechnologyEscherichia coli
Owner:HUNAN UNIV OF SCI & TECH
Application of lignin flavanonol in preparation of antiviral drugs
InactiveCN101548970AEasy to prepareLow costOrganic active ingredientsDigestive systemBenzopyroneKetone
The invention relates to application of lignin flavanonol in preparation of antiviral drugs, particularly, the invention relates to a chemical compound as formula (I), (+-)-2-[2,3-dihydro-2-(3,5-dimethoxy-4-hydroxy phenyl)-3-hydroxymethyl-1,4-benzodioxane-5]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyrone-4-ketone, or use of its medicinal salt used for preparing drugs for repressing herpes simplex virus and / or influenza virus. Pharmacological experiments in vitro shows that the chemical compound (I) can not only repress growth of herpes simplex virus HSV-1, HSV-2 obviously, and repress influenza virus effectively. And so it is expected to be develpoed as anti-infective agents for curing I / II type herpes simplex virus, or develpoed as viral influenza drugs infected by IV A virus.
Owner:WENZHOU MEDICAL UNIV +1
Flavanonol compounds and their production method and use
InactiveCN1990482BHas a growth effectGrowth inhibitory effectOrganic active ingredientsAntimycoticsAntifungalAutoimmune condition
The invention relates to a novel 5, 6, 7- tri-oxygen Dihydroflavonol, shown in formula (1), the preparation method and its application. The compound can be used to prepare drug combination that can prevent hepatitis b, protect liver, treat fungal infection, prevent diseases that generates free radical during disease change including inflammation, autoimmune diseases, radiotherapy sequela, tumor, myocardial ischemia, cardiac hypertrophy, aging, allergic reaction and atherosclerosis. There are various preparations for said drug combination.
Owner:ZHEJIANG HISUN PHARMA CO LTD
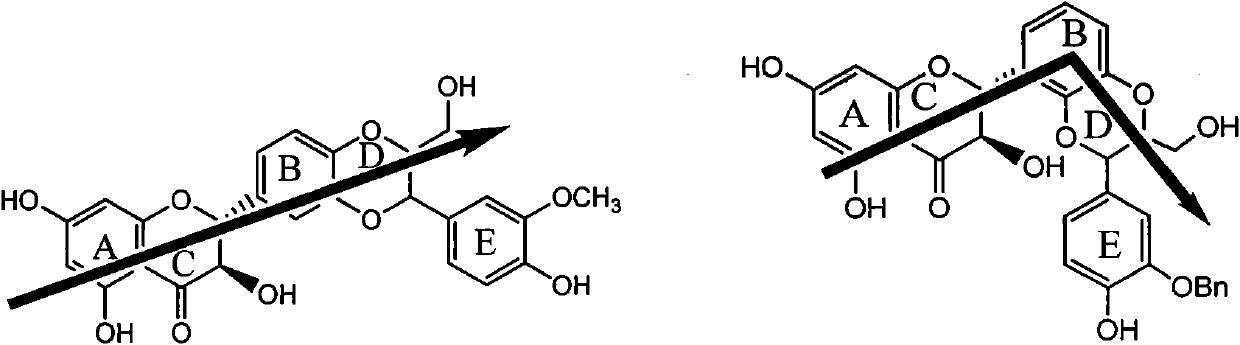
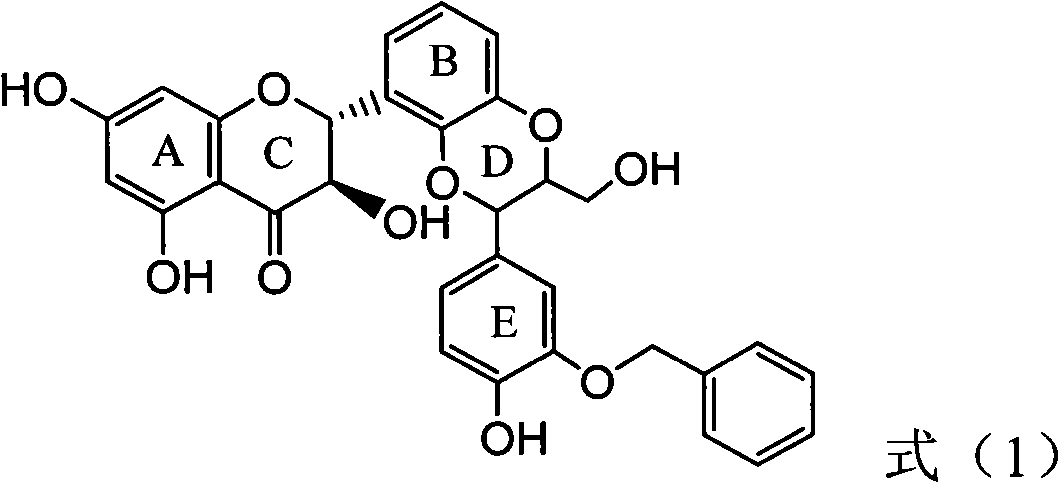
Application of flavanonol lignanoid in preparing antiviral hepatitis B medicine
InactiveCN101829100AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of flavanonol lignanoid in preparing an antiviral hepatitis B medicine, in particular to application of angle flavanolignan or pharmaceutically acceptable salts thereof in preparing a medicine which can be used for eliminating hepatitis B e antigen HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases. The flavanolignan has a certain HBeVg activity inhibition, the HBeAg elimination intensity of the flavanolignan is higher than that of a positive control first-line medicine as lamivudine in light concentration and is equivalent to that of alpha-interferon of 1000 unit / ml, and meanwhile, under the concentration of 20 microgram / ml, the flavanolignan displays an inhibition ratio which is larger than 45 percent for HBV DNA. The pharmacodynamics result indicates the application of the flavanolignan or the pharmaceutically acceptable salts thereof in preparing the medicine as expected for eliminating hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
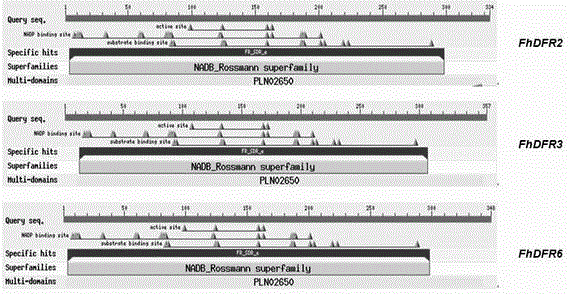
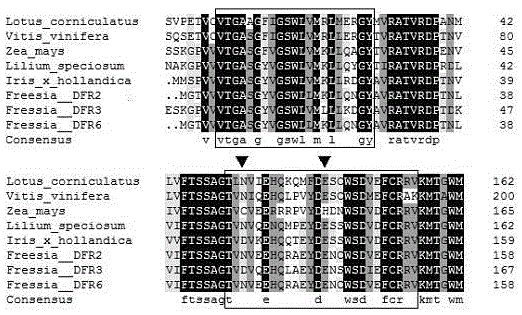
CDNA of Freesia refracta Klatt flavanonol-4-reductase genes
The new cDNA of three dihydroflavonol-4-reductase (DFR) genes is obtained through cloning from red flower Freesia refracta Klatt petals for the first time in the invention, and the nucleotide sequence of the cDNA and an amino acid sequence obtained from the nucleotide sequence are determined. Gene expression of the three genes has obvious correlation with anthocyanin synthesis in the coloring process of Freesia refracta Klatt flowers; and additionally, after eukaryotic expression vectors of the three FhDFRs are transferred to Arabidopis thaliana tt3 mutants, phenotypes of seedlings and seed coats are recovered.
Owner:NORTHEAST NORMAL UNIVERSITY
Method for preparing general flavone and total alkaloid of sophora flavescens simultaneously
The invention discloses a method for simultaneously preparing total flavone extract and total alkaloid extract of Radix sophorae flavescentis. Total flavone extract mainly comprises flavanones (flavanonols) such as norkurarinone, kurarinone, and isokurarinon and other flaconoids such as trifolirhizin, xanthohumol, and kuraridin. Total alkaloid extract mainly comprises matrine n-oxide, N-oxysophocarpine, matrine, sophocarpine, sophoridine, and sophoramine. Total flavone extract and total alkaloid extract can be extracted through one or more of methods of solvent-extraction, solvent extraction,macroporous adsorbent resin, extraction by supercritical fluid, column chromatography, and liquid-liquid countercurrent distribution chromatography. Flavonoid content in total flavone extract is 5-100wt%, wherein the content of norkurarinone, xanthohumol, kuraridin, and trifolirhizin occupies 1-100wt%.Alkaloid content in total alkaloid extract is 5-100wt%, wherein the content of matrine n-oxide, N-oxysophocarpine, matrine and sophocarpine occupies 5-100wt%.
Owner:石任兵 +1
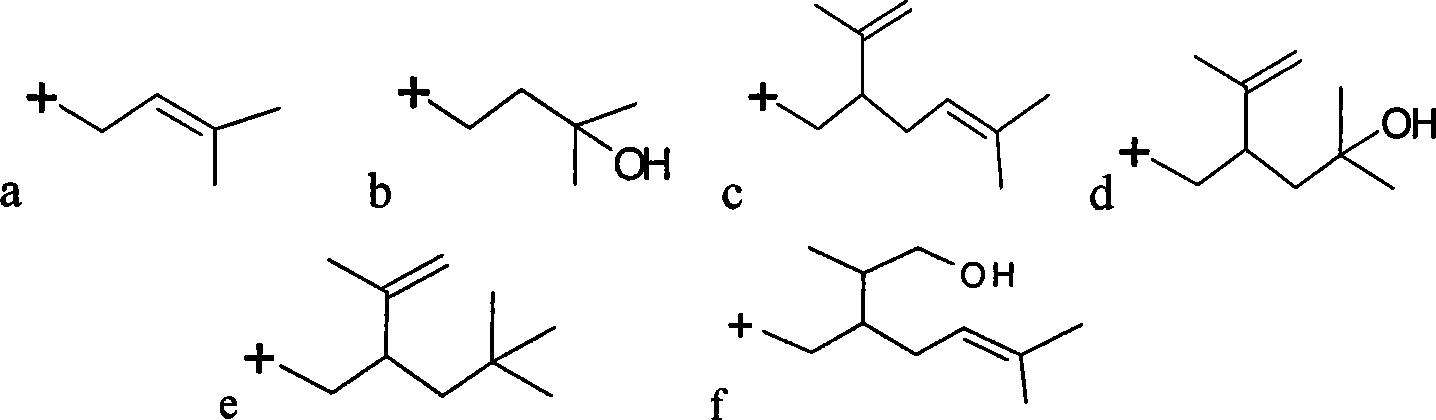
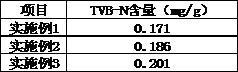
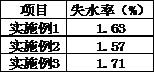
A kind of treatment agent for cryopreservation and quick-freezing of Chinese tube whip shrimp and its application
ActiveCN107467164BReduce water lossImprove frozen qualityFood freezingAcidic food ingredientsArbutinBetaine
The invention discloses a deeply-cold quick-frozen treatment agent for solenocera crassicornis. The deeply-cold quick-frozen treatment agent is prepared from the following components in percentage by mass: 5-10% of glycerine, 1-3% of carnosol, 0.01-0.03% of transglutaminase, 1-3% of cysteine, 0.5-1% of betaine, 0.1-0.3% of arbutin, 0.1-0.3% of dihydroflavonol, 0.1-0.3% of cuminic acid, 0.5-1% of konjac glucomannan and the balance of water. The deeply-cold quick-frozen treatment agent for solenocera crassicornis is reasonable and scientific in formula, is good in fresh-keeping and color-protection effect, can prevent the situation that solenocera crassicornis heads come off from solenocera crassicornis bodies, and can effectively restrain nigrities. The invention further discloses an application of the deeply-cold quick-frozen treatment agent for solenocera crassicornis in deeply-cold quick-frozen solenocera crassicornis. The solenocera crassicornis is soaked in the deeply-cold quick-frozen treatment agent for at least 10min, then draining is performed, and liquid nitrogen deeply-cold quick-frozen treatment is performed, so that the method is simple in steps and high in maneuverability.
Owner:MARINE FISHERIES RES INST OF ZHEJIANG
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
InactiveCN101822664BConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
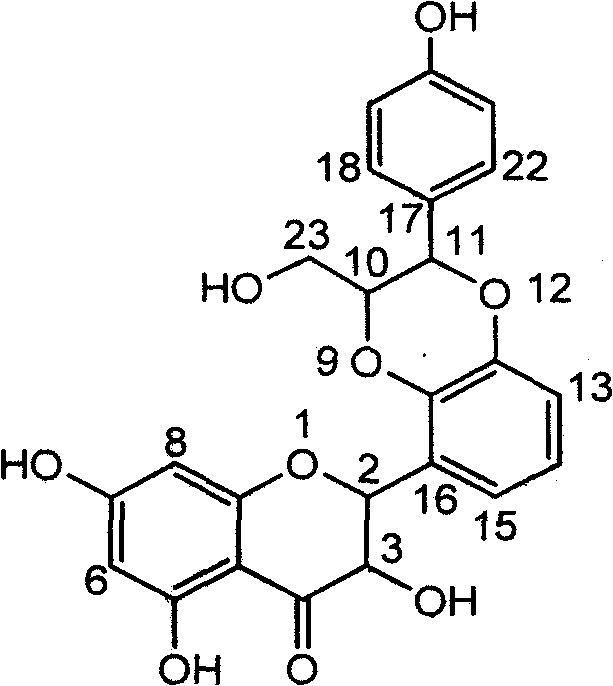
Medicinal application of flavanonol lignan in preparation glycosidase inhibitors
InactiveCN102000064AConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderLignanDiabrezide
The invention relates to medicinal application of flavanonol lignan in preparing glycosidase inhibitors, in particular to application of B-ring dioxane flavanonol lignan or pharmaceutically acceptable salts thereof in preparation medicines for inhibiting alpha-glucosidase and preventing type 2 diabetes. The flavone lignan has extremely obvious activity of inhibiting the alpha-glucosidase and has the activity strength of inhibiting the alpha-glucosidase at the concentration of 40 micrograms / milliliter of 93 percent; and a half inhibitory concentration test shows that: the strength of the flavone lignan of inhibiting the alpha-glucosidase is 1.2 times that of a positive contrast medicine acarbose. A pharmacodynamic result shows that the flavone lignan or the pharmaceutically acceptable salts thereof can be expected to serve as the glycosidase inhibitors, particularly serve as medicines for preventing the type 2 diabetes.
Owner:DALI UNIV
Medicinal application of flavanonol lignan in preparation glycosidase inhibitors
InactiveCN102000064BConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderDiabrezideLignan
The invention relates to medicinal application of flavanonol lignan in preparing glycosidase inhibitors, in particular to application of B-ring dioxane flavanonol lignan or pharmaceutically acceptable salts thereof in preparation medicines for inhibiting alpha-glucosidase and preventing type 2 diabetes. The flavone lignan has extremely obvious activity of inhibiting the alpha-glucosidase and has the activity strength of inhibiting the alpha-glucosidase at the concentration of 40 micrograms / milliliter of 93 percent; and a half inhibitory concentration test shows that: the strength of the flavone lignan of inhibiting the alpha-glucosidase is 1.2 times that of a positive contrast medicine acarbose. A pharmacodynamic result shows that the flavone lignan or the pharmaceutically acceptable salts thereof can be expected to serve as the glycosidase inhibitors, particularly serve as medicines for preventing the type 2 diabetes.
Owner:DALI UNIV
Application of flavanonol lignanoid in preparing antiviral hepatitis B medicine
InactiveCN101829100BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of flavanonol lignanoid in preparing an antiviral hepatitis B medicine, in particular to application of angle flavanolignan or pharmaceutically acceptable salts thereof in preparing a medicine which can be used for eliminating hepatitis B e antigen HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases. The flavanolignan has a certain HBeVg activity inhibition, the HBeAg elimination intensity of the flavanolignan is higher than that of a positive control first-line medicine as lamivudine in light concentration and is equivalent to that of alpha-interferon of 1000 unit / ml, and meanwhile, under the concentration of 20 microgram / ml, the flavanolignan displays an inhibition ratio which is larger than 45 percent for HBV DNA.The pharmacodynamics result indicates the application of the flavanolignan or the pharmaceutically acceptable salts thereof in preparing the medicine as expected for eliminating hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of lignin flavanonol in preparation of antiviral drugs
InactiveCN101548971BEnhanced inhibitory effectHigh yieldOrganic active ingredientsDigestive systemChemical synthesisBenzopyrone
The invention relates to application of lignin flavanonol in preparation of antiviral drugs, particularly, the invention relates to a chemical compound as formula (I), (+-)-2-[2,3-dihydro-2-(3,5-dimethoxy-4-hydroxy phenyl)-3-hydroxymethyl-1,4-benzodioxane-5]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyrone-4-ketone, or use of its medicinal salt used for preparing drugs for repressing virus infectioncaused by herpes simplex virus HSV-1 and / or drugs for curing oral ulcer. The chemical compound is prepared by chemical synthesis, pharmacological experiments shows that, the chemical compound has powerful inhibitory action to the herpes simplex virus HSV-1, and the half inhibitory concentration value IC[50] is 39.41 mg / mL.
Owner:WENZHOU MEDICAL UNIV +1
Gene sequence of flavanonol 4-reductase synthesized by anthocyanins of eggplant
The invention relates to a gene sequence of flavanonol 4-reductase synthesized by anthocyanins of an eggplant, belonging to the technical field of biology. The gene sequence has a nucleotide sequence as shown in 1st-1149th places of nucleotide in SEQ ID NO. 1; and another sequence has a nucleotide sequence as shown in 1st-1149th places of the nucleotide in SEQ ID NO. 3. In the invention, an eggplant variety has significant characteristics on the aspects of high anthocyanins, water stress resistance, uvioresistant protection, and the like, can be obtained; substances, receptors, inhibitors or antagonists, and the like relatively interacted with the flavanonol 4-reductase can be screened through various conventional screening methods by utilizing a flavanonol 4-reductase gene of the eggplant.
Owner:SHANGHAI JIAO TONG UNIV
Novel flavone reductase and application thereof
The invention relates to novel flavonoid reductase and application thereof, in particular to flavonoid reductase capable of catalyzing a flavonoid compound / flavonol compound to be reduced into a flavanone compound / flavanol compound. And catalyzing the flavanone compound / flavanonol compound to be oxidized into a flavonoid compound / flavanonol compound.
Owner:艾博锐克生物科技(山东)有限公司
Extraction separation method of a flavone component based on graphene
The present invention refers to the technical field of flavone component extraction, and provides an extraction separation method of a flavone component based on amination graphene. The flavone components comprise flavones, flavanols, isoflavones, flavanones, flavanonols, flavanones, anthocyanidins, chalcones, and chromones etc. The extraction separation method is adsorption extraction, and amination graphene is taken as a medium of adsorption extraction. The extraction separation method of the flavone components based on amination graphene is superior in separation speed and product purity, low cost and convenient operation.
Owner:SHENZHEN XIHAN MEDICAL & HEALTH ENVIRONMENTAL PROTECTION CO LTD
Method for Extracting Flavonoids with Antioxidant Activity and 3,4',7-Trihydroxydihydroflavonol from Sumac Flour
ActiveCN105669627BAvoid decompositionHigh extraction rateOrganic chemistryAntinoxious agentsAdditive ingredientFlavonols
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
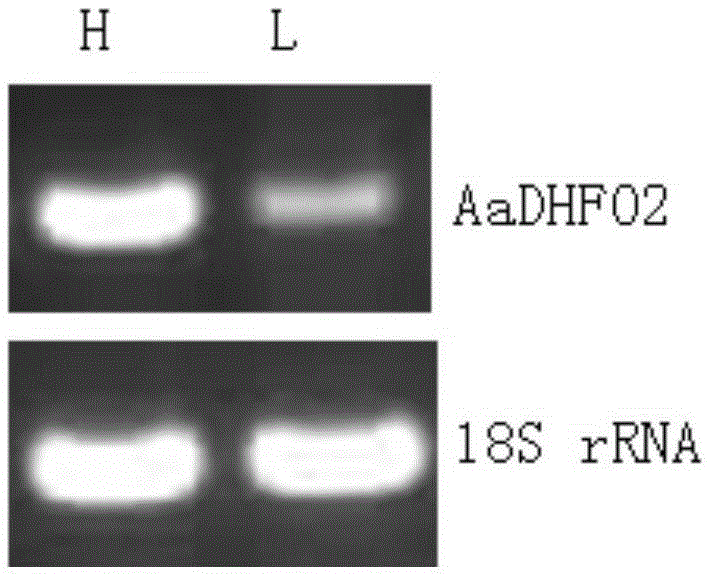
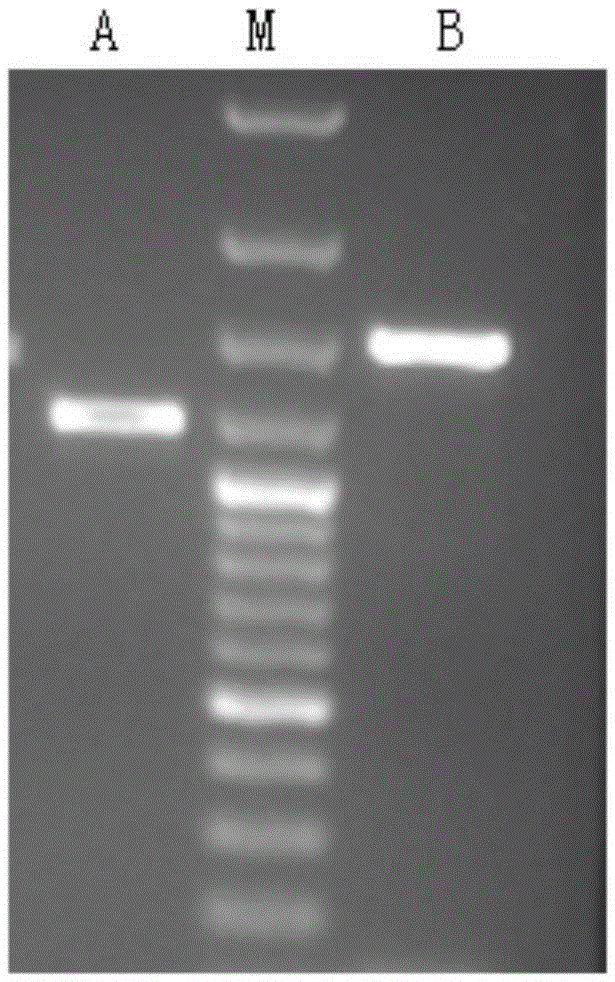
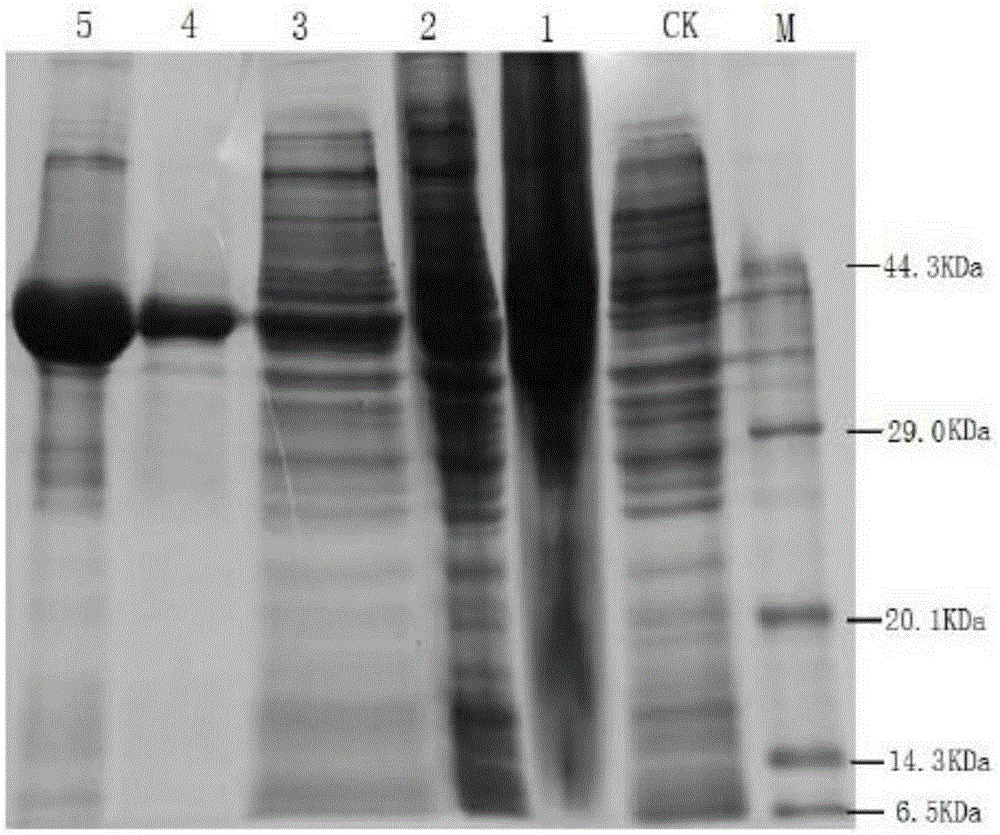
Artemisia annua flavanonol oxidase gene AaDHFO2 as well as encoding protein and application thereof
InactiveCN105296510AAvoid difficultiesLow costOxidoreductasesFermentationNucleotideProkaryotic expression
The invention provides an artemisia annua flavanonol oxidase gene AaDHFO2. A nucleotide sequence of the artemisia annua flavanonol oxidase gene AaDHFO2 is shown in SEQ ID No.1, and an amino acid sequence of the encoding protein is shown in SEQ ID No.2. The artemisia annua flavanonol oxidase gene AaDHFO2 can catalyze flavanonol to form flavonol and provides a new optional approach for generation of flavonol through in-vitro catalysis of flavanonol and regulation of artemisia annua component in artemisia annua; besides, recombinant AaDHFO2 is prepared through prokaryotic expression, the difficulty of directly separating the protein from the artemisia annua is overcome, and the cost is reduced.
Owner:HUNAN AGRICULTURAL UNIV
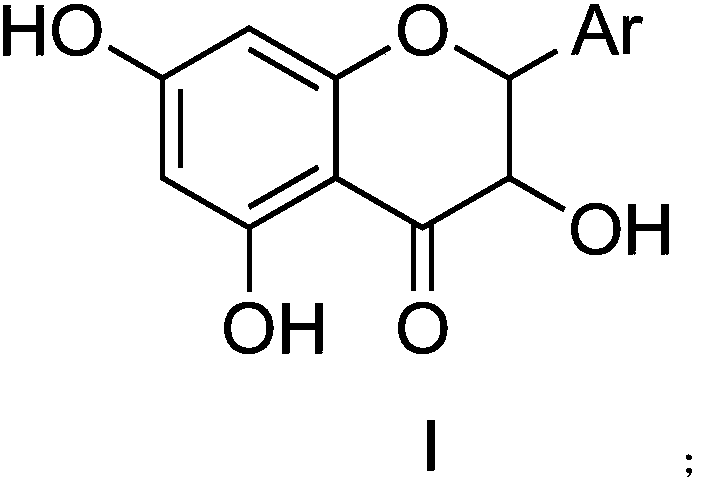
Flavanonol compound containing N aromatic heterocycte and preparation method and application thereof
ActiveCN107629040AGood anti-inflammatory activityHigh activityOrganic chemistryAntipyreticChemical compoundTaxifolin
The invention discloses a flavanonol compound containing N aromatic heterocycte, which has a following structure in a specification, wherein an Ar substituent in a formula I is shown as one in the specification, and the substituted position of the compound comprises all the substituted positions on the N aromatic heterocycte. The novel flavanonol compound containing N aromatic heterocycte has strong anti-inflammatory activity, and compared with positive contrast indomethacin and taxifolin, the activity is equal or better. The flavanonol compound can be used for preparing the anti-inflammatorymedicines.
Owner:HUBEI UNIV OF CHINESE MEDICINE
Pharmaceutical use of flavanonol natural products
InactiveCN110384695AReduce Pathology ScoreImprove transportation capacityOrganic active ingredientsDigestive systemDiseaseNatural product
The invention relates to application of a flavanonol compound, namely silibinin, to treatment of a metabolic disease, namely non-alcoholic steatohepatitis (NASH).
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Application of lignin flavanonol in preparation of antiviral drugs
InactiveCN101548971AEasy to prepareLow costOrganic active ingredientsDigestive systemChemical synthesisBenzopyrone
The invention relates to application of lignin flavanonol in preparation of antiviral drugs, particularly, the invention relates to a chemical compound as formula (I), (+-)-2-[2,3-dihydro-2-(3,5-dimethoxy-4-hydroxy phenyl)-3-hydroxymethyl-1,4-benzodioxane-5]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyrone-4-ketone, or use of its medicinal salt used for preparing drugs for repressing virus infection caused by herpes simplex virus HSV-1 and / or drugs for curing oral ulcer. The chemical compound is prepared by chemical synthesis, pharmacological experiments shows that, the chemical compound has powerful inhibitory action to the herpes simplex virus HSV-1, and the half inhibitory concentration value IC[50] is 39.41 mg / mL.
Owner:WENZHOU MEDICAL UNIV +1
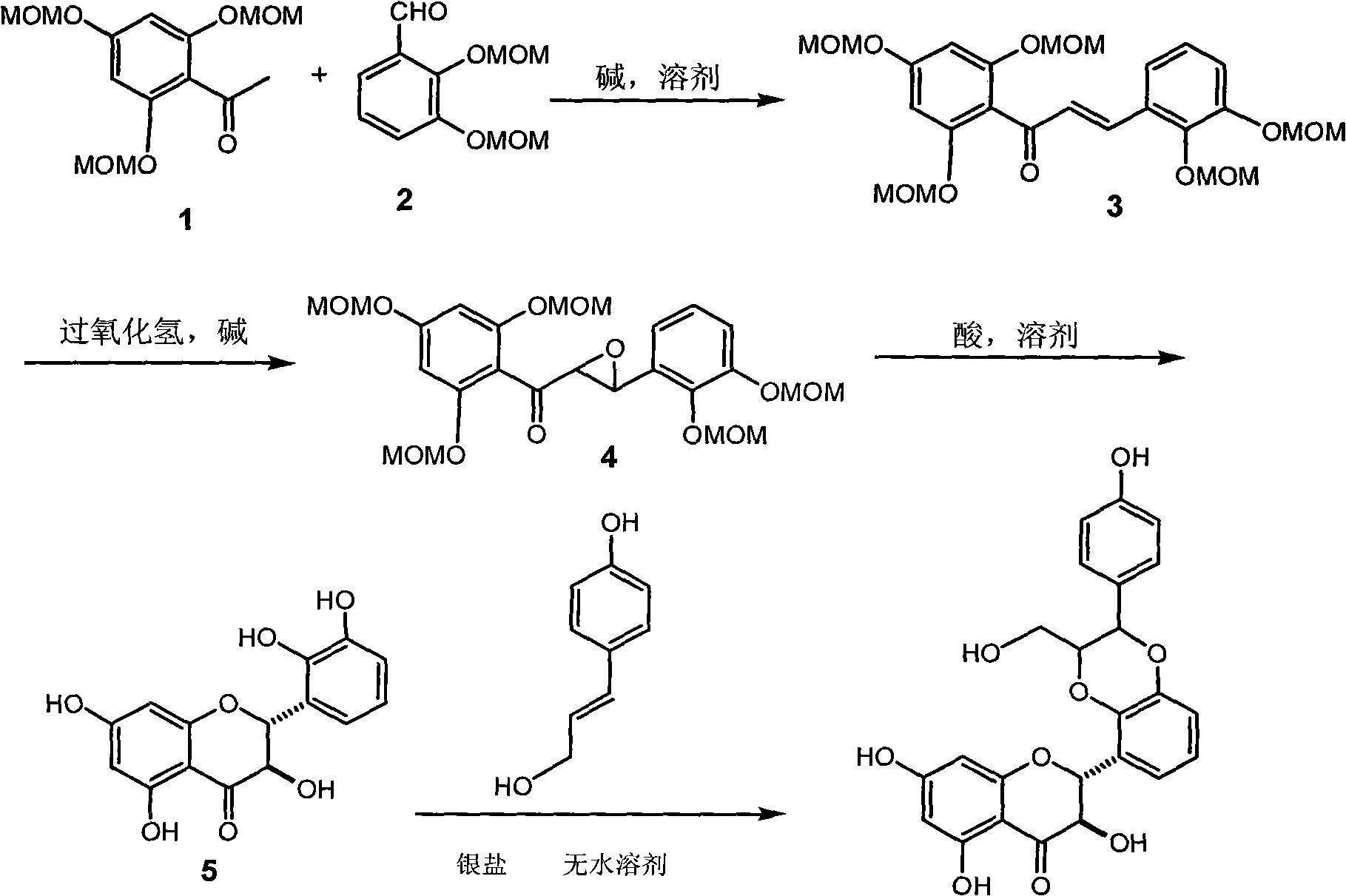
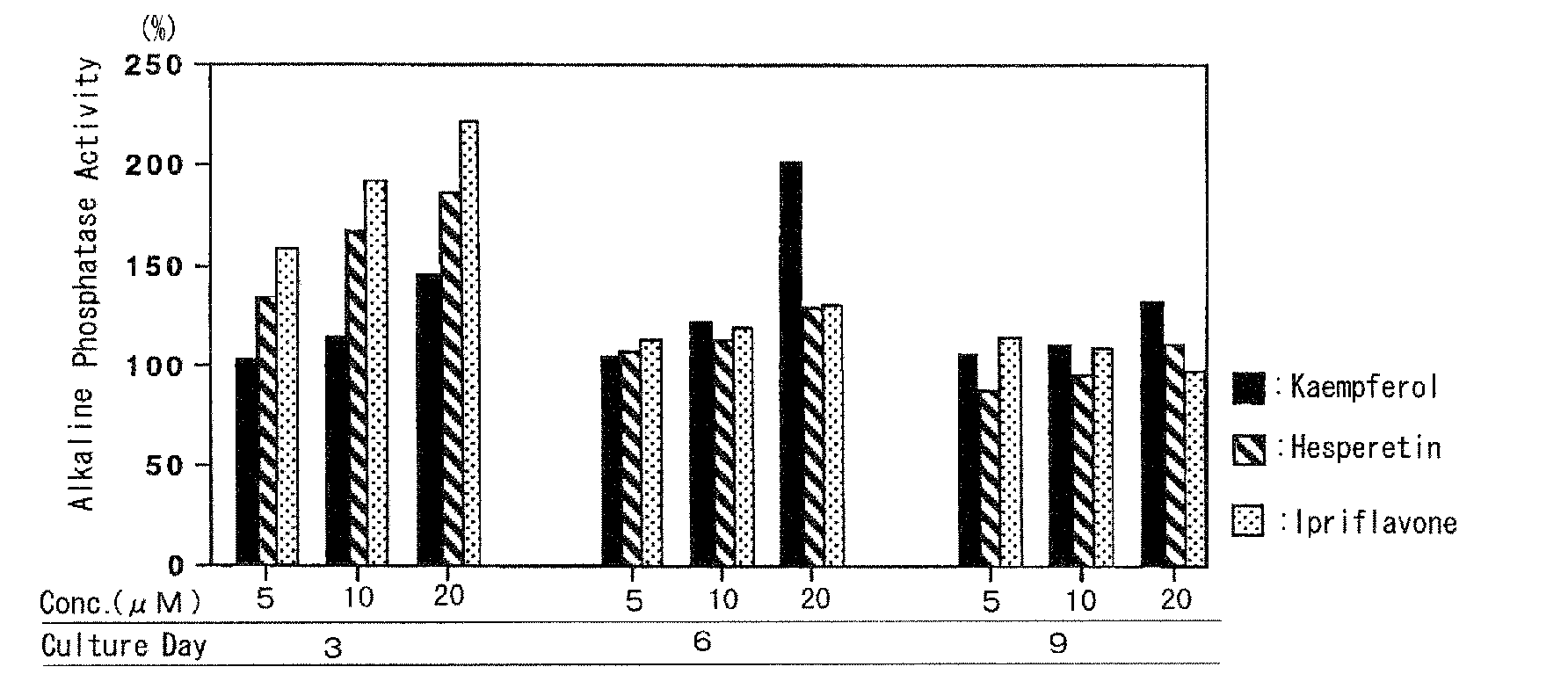
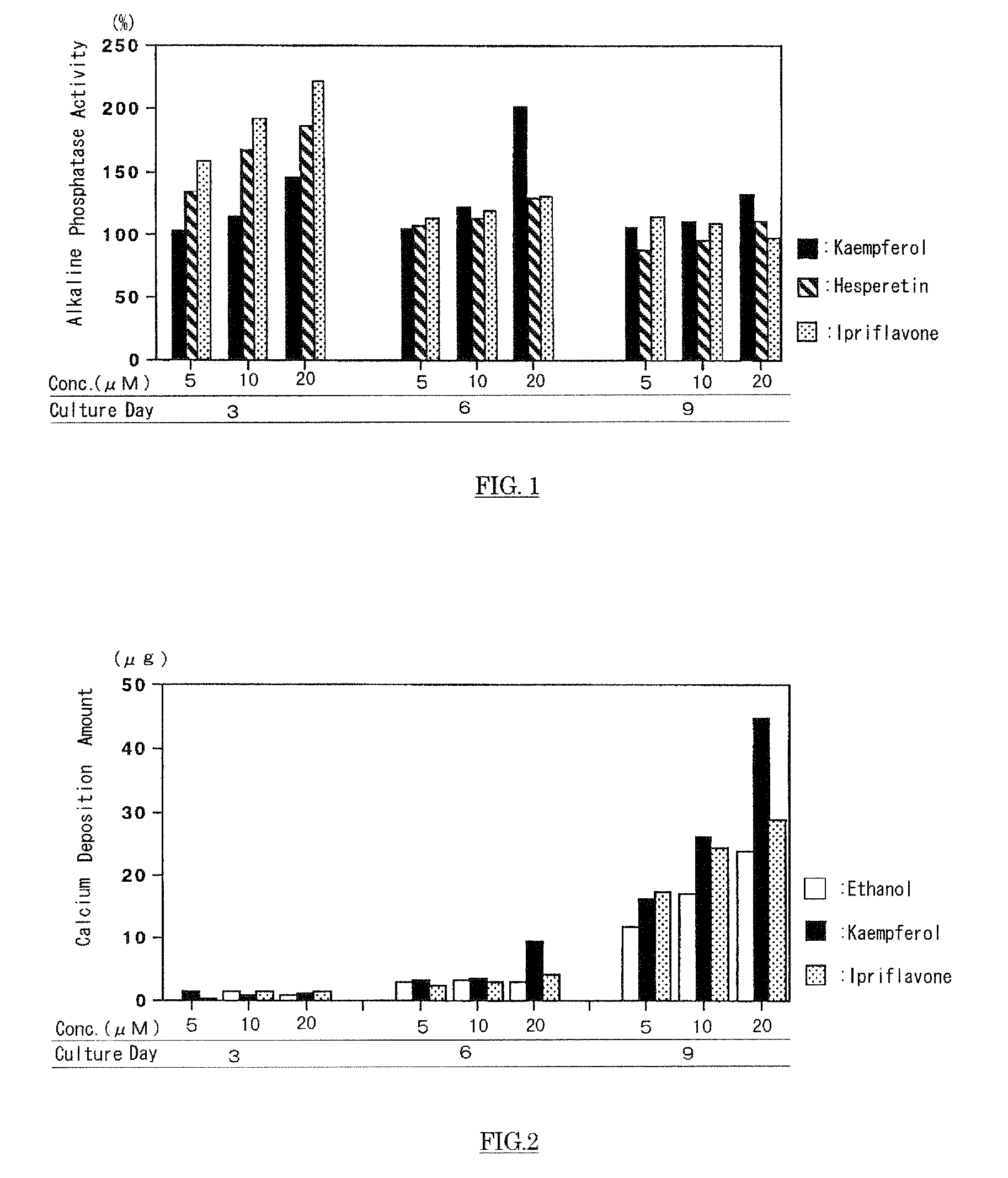
Agent For Strengthening Calcium Containing Tissue and Use Thereof
InactiveUS20090075908A1Quality improvementIncrease depositionBiocideCosmetic preparationsFlavanoneFlavonols
The present invention has an object to provide an agent for strengthening calcium-containing tissues, which can be safely applied; and its use: The present invention solves the object by providing an agent for strengthening calcium-containing tissues, which comprises one or more flavones, flavonols, flavanones, flavanonols, anthocyanidins, flavanols, chalcones, and aurones.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com