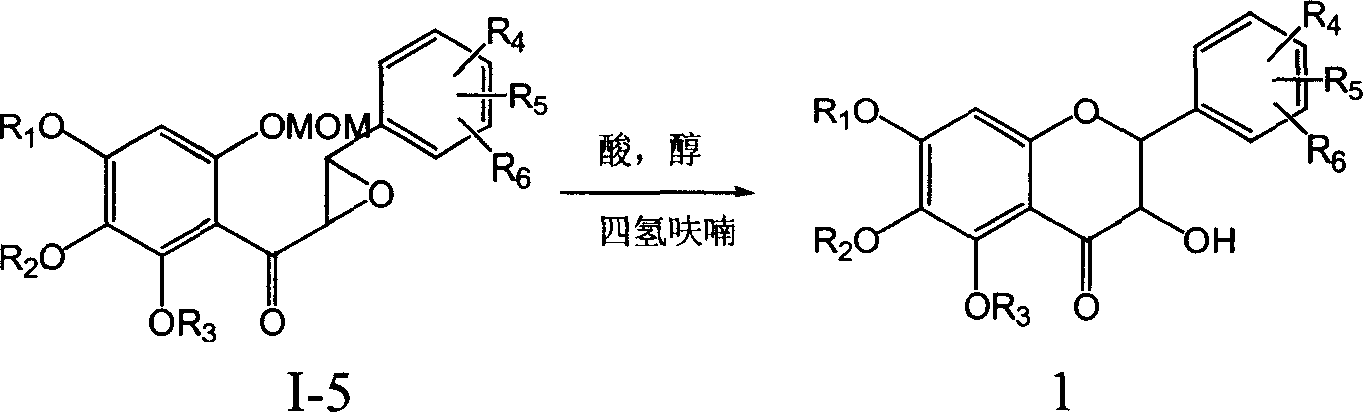
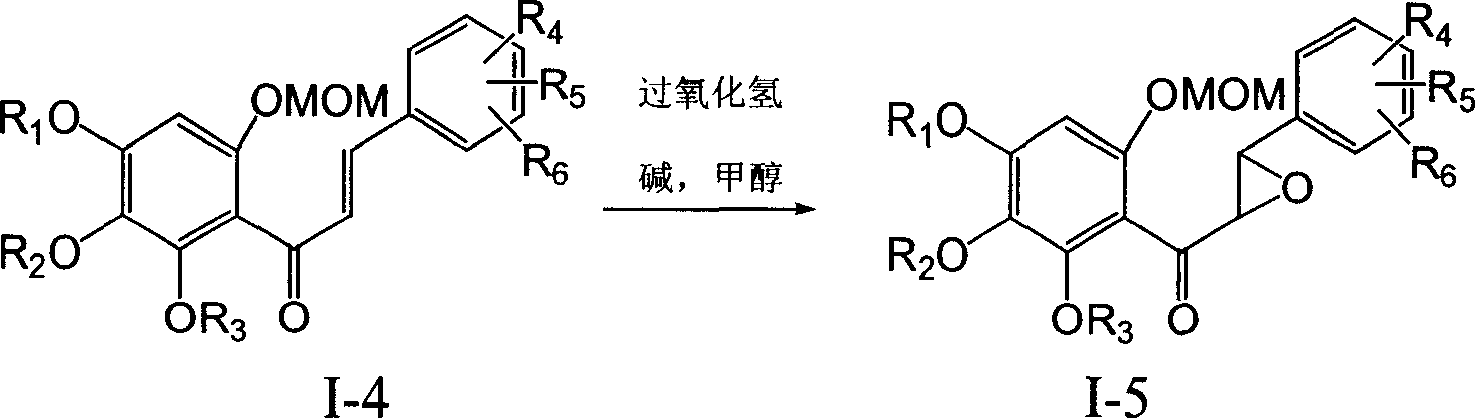
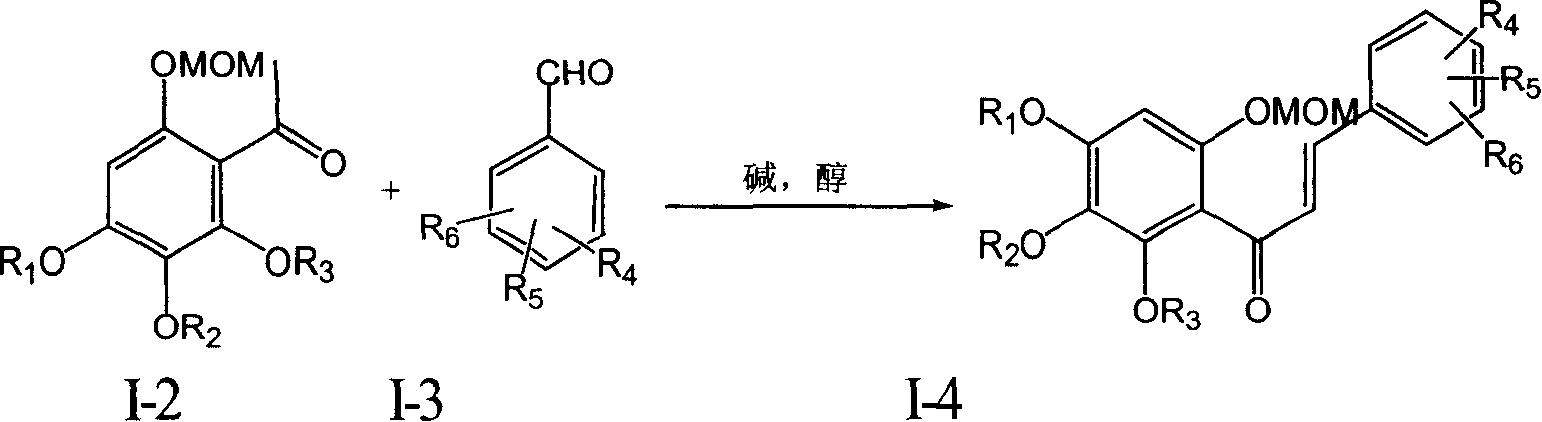
Flavanonol compounds and their production method and use
A technology of dihydroflavonol and trimethoxydihydroflavonoids, which is used in medical preparations containing active ingredients, drug combinations, organic chemistry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of compound I-2-1 (6-methoxymethoxy-2,3,4-trimethoxyacetophenone)
[0051]
[0052] 1.5 grams of 6-hydroxyl-2,3,4-trimethoxyacetophenone was dissolved in 20 milliliters of dichloromethane, and then 1.5 grams of sodium hydroxide was dissolved to obtain 20 milliliters of water and 0.4 gram of tetrabutylammonium bromide, Stir and cool down to 0°C, slowly add 1.5 ml of distilled chloromethyl ether, stir at room temperature for 12 hours, let stand, separate the organic layer, extract the aqueous layer with 10 ml of dichloromethane three times, combine them, and wash with saturated saline After washing, drying over sodium sulfate, filtering, and concentration, the residue was subjected to column chromatography to obtain 1.4 g of light yellow oil, with a yield of 80%. Rf (petroleum ether / ethyl acetate=3:1): 0.21, 1 H NMR (400MHz, deuterated chloroform) δ: 2.56(s, 3H, CH 3 ), 3.78(s, 3H, OCH 3 ), 3.85(s, 3H, OCH 3 ), 3.90 (s, 3H, OCH 3 ), 5.15(...
Embodiment 2
[0053] Embodiment 2: Preparation of compound I-2-2 (2,4-dimethoxy-3,6-dimethoxymethoxyacetophenone):
[0054]
[0055] 1.76 grams of 3,6-dihydroxy-2,4-dimethoxyacetophenone was dissolved in 20 milliliters of dichloromethane, and then 1.5 grams of sodium hydroxide was added to obtain 20 milliliters of water and 0.4 grams of tetrabutyl bromide ammonium, stirred and cooled to 0°C, slowly added 1.5 ml of distilled chloromethyl ether, stirred at room temperature for 12 hours, stood still, separated the organic layer, extracted three times with 10 ml of dichloromethane and combined the aqueous layer with saturated Washed with brine, dried over sodium sulfate, filtered, concentrated, and the residue was subjected to column chromatography to obtain 1.63 g of light yellow oil with a yield of 82%. Rf (petroleum ether / ethyl acetate=3:1): 0.30, 1 H NMR (400MHz, deuterated chloroform) δ: 2.48(s, 3H, CH 3 ), 3.60 (s, 3H, OCH 3 ), 3.85(s, 3H, OCH 3 ), 3.86(s, 3H, OCH 3 ), 5.05(s, 2H,...
Embodiment 3
[0059] Embodiment 3: Preparation of compound I-4-1 (6,4'-dimethoxymethoxy-2,3,4-trimethoxychalcone)
[0060]
[0061] 1.6 grams of I-2-1 were dissolved in 60 milliliters of methanol with 0.5 grams of potassium hydroxide, then 1.5 grams of p-methoxybenzaldehyde was added, and after stirring at room temperature for 8 hours, the solvent was evaporated under reduced pressure, and the residue was Add 30 milliliters of water, extract three times with 20 milliliters of ethyl acetate, combine the organic phases and wash with saturated brine, dry over sodium sulfate, filter, and concentrate the filtrate, and the residue is subjected to injection chromatography to obtain 2.1 grams of light yellow oil, with a yield of 84 %.
[0062] Rf (petroleum ether / ethyl acetate=3: 1)=0.13; 1 H NMR (400MHz, deuterated chloroform) δ: 3.41(s, 3H, OCH 3 ), 3.47 (s, 3H, OCH 3 ), 3.84(s, 3H, OCH 3 ), 3.85(s, 3H, OCH 3 ), 3.90 (s, 3H, OCH 3 ), 5.10 (s, 2H, OCH 2 O), 5.20(s, 2H, OCH 2 O), 6.57(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com