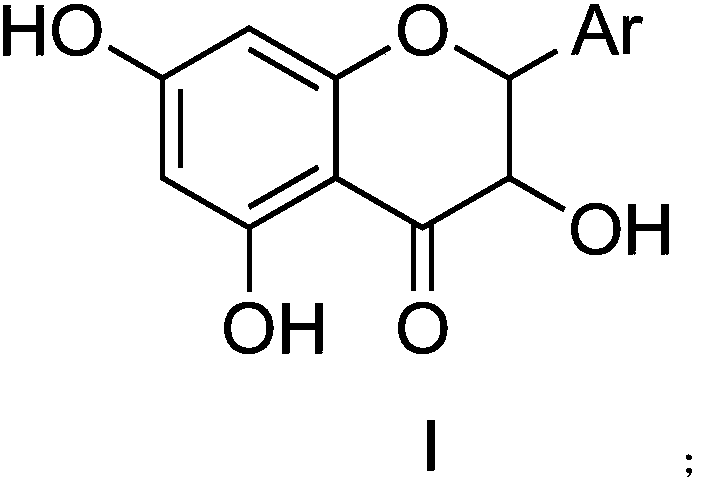
Flavanonol compound containing N aromatic heterocycte and preparation method and application thereof
A technology of dihydroflavonols and aromatic heterocycles, applied in organic chemistry, drug combination, antipyretics, etc., can solve problems such as side reactions and achieve strong anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: 2-(3 / Total Synthesis of -pyridyl)-3,5,7-trihydroxychroman-4-one
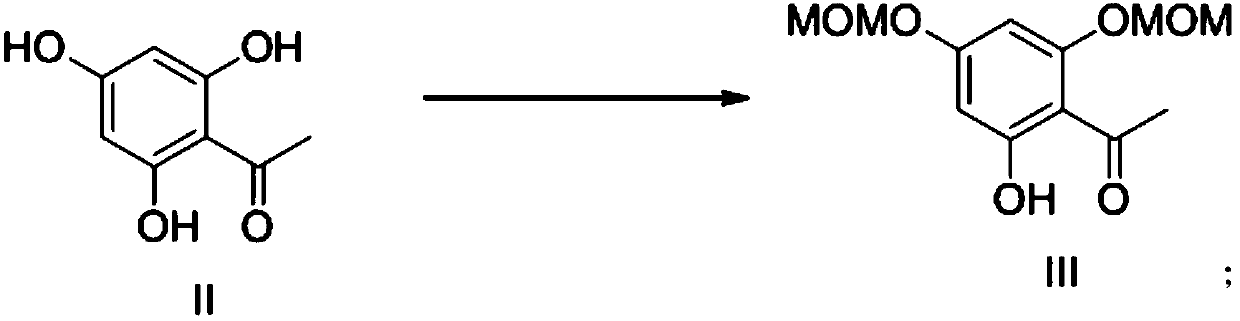
[0024] (1) Preparation of 2,4-dimethoxymethoxy-6-hydroxyacetophenone
[0025]
[0026] Dissolve 1g of 2,4,6-trihydroxyacetophenone in 30ml of acetone, add 2.88g of anhydrous potassium carbonate, reflux and stir for 30min, cool slightly, add 1.13ml of chloromethyl ether dropwise, continue to reflux, TLC plate detects the reaction After completion, the reaction was quenched by adding water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain 1.32 g of 2,4-dimethoxymethoxy-6-hydroxyacetophenone with a yield of 86.62%.
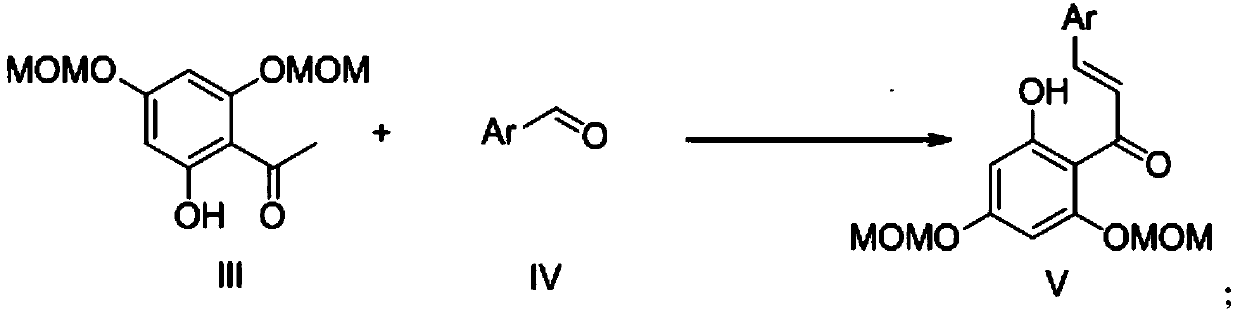
[0027] (2) 3-pyridyl-2 / ,4 / -Dimethoxymethoxy-6 / - Preparation of hydroxychalcone
[0028]
[0029] Dissolve 0.72g of 3-pyridinecarbaldehyde in 25mL of anhydrous methanol, add 5.20mL of 50% sodium hydroxide solution under ice-cooling conditions, stir, and then add 1g of 2,4-dimethoxymethoxy-6-hydroxyacet...
Embodiment 2
[0036] Embodiment 2: 2-(3 / Anti-inflammatory activity of -pyridyl)-3,5,7-trihydroxychroman-4-one (a)
[0037] (1) Establishment of inflammation model
[0038] Take the cultured cells in the logarithmic growth phase, digest with trypsin, and take 1% fetal bovine serum H-DMEM medium to make 8×10 4 cells / ml single cell suspension, uniformly inoculated in 96-well plate with 100 μl per well, placed at 37°C, 5% CO 2 Culture in an incubator, and discard the old medium after 12 hours. The complete medium containing LPS (1 μg / mL) was added, and the culture was continued for 24 hours, and the establishment of the cell inflammation model was completed.
[0039] (2) ELISA was used to detect the levels of inflammatory factors IL-1β, IL-6 and TNF-ɑ in the culture medium of RAW264.7 cells induced by LPS
[0040] Take the cultured cells in the logarithmic growth phase, digest with trypsin, and take 1% fetal bovine serum H-DMEM medium to make 8×10 4 cells / ml single cell suspension, unifo...
Embodiment 3
[0045] Embodiment 3: 2-(2 / Total Synthesis of -furyl)-3,5,7-trihydroxychroman-4-one
[0046] (1) Preparation of 2,4-dimethoxymethoxy-6-hydroxyacetophenone
[0047]
[0048] Dissolve 1g of 2,4,6-trihydroxyacetophenone in 30ml of acetone, add 2.88g of anhydrous potassium carbonate, reflux and stir for 30min, cool slightly, add 1.13ml of chloromethyl ether dropwise, continue to reflux, TLC plate detects the reaction After completion, the reaction was quenched by adding water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain 1.32 g of 2,4-dimethoxymethoxy-6-hydroxyacetophenone with a yield of 86.62%.
[0049] (2) 2-furyl-2 / ,4 / -Dimethoxymethoxy-6 / - Preparation of hydroxychalcone
[0050]
[0051] Dissolve 0.70g of 2-furfuraldehyde in 25mL of anhydrous methanol, add 5.20mL of 50% sodium hydroxide solution under ice-cooling conditions, stir, and then add 1g of 2,4-dimethoxymethoxy-6-hydroxyphenylethyl k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com