Chitosan oligosaccharide-N-linalool copolymer and preparation method and application thereof
A technology of chitosan oligosaccharide and linalool is applied in the field of antibacterial agents and anti-inflammatory agents, which can solve the problem of low activity and achieve the effects of enhancing antibacterial activity, simple preparation process and purification process, and good anti-inflammatory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
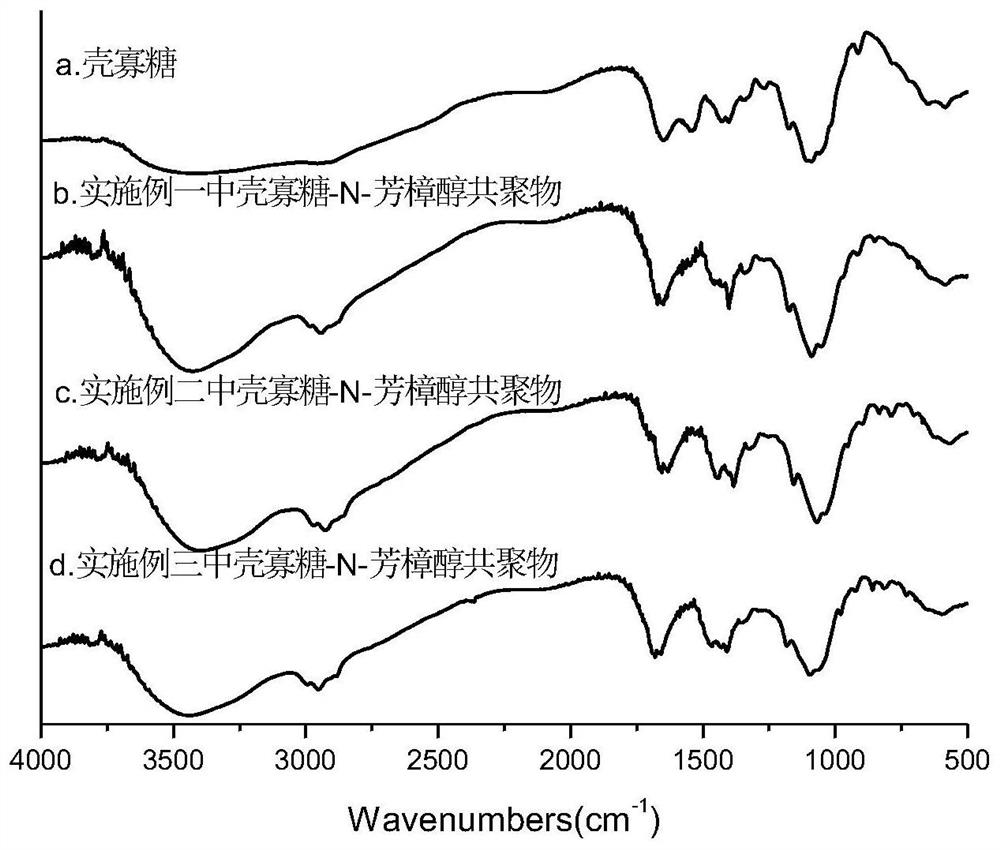
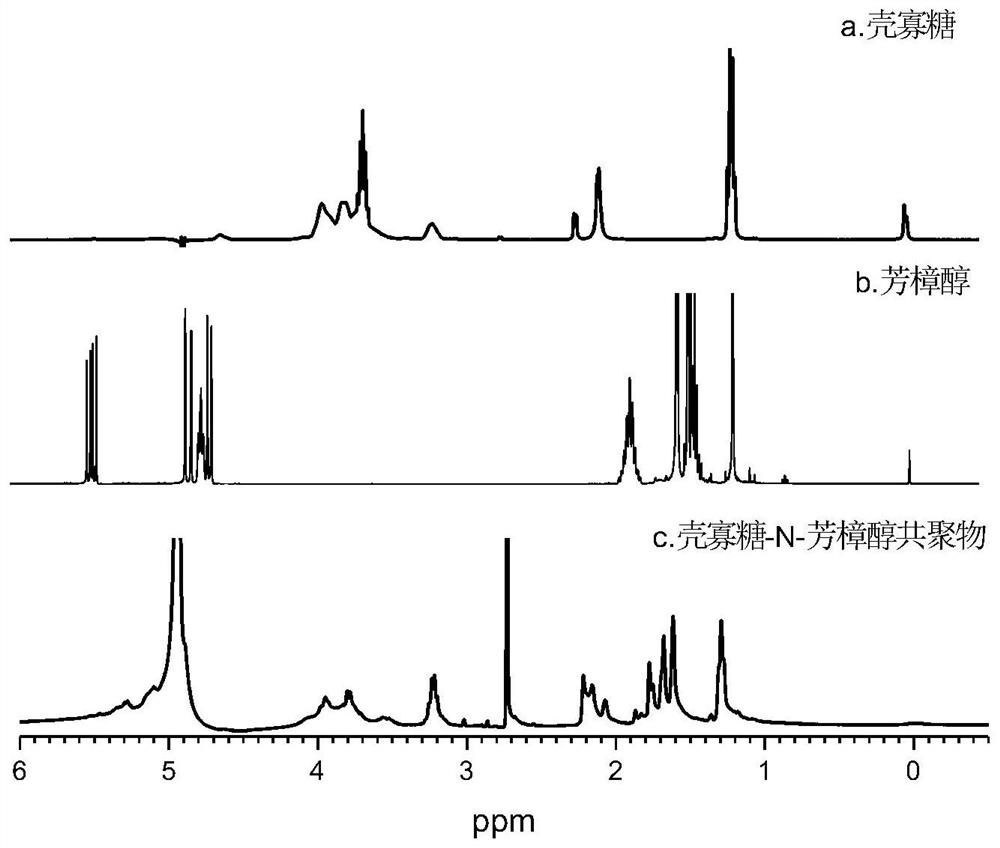
Image
Examples
Embodiment 1
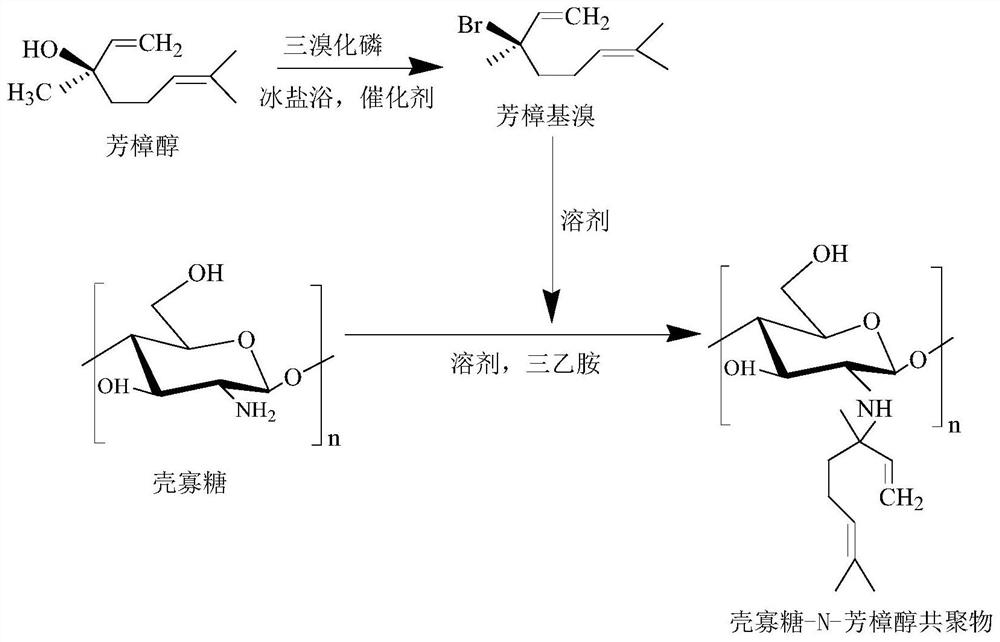
[0042] Embodiment 1: Synthesis of chitosan oligosaccharide-N-linalool copolymer
[0043] 1. Preparation of linalyl bromide
[0044] First, dissolve 5 mL of linalool and 2 mL of phosphorus tribromide with 30 mL of anhydrous ether, respectively, add linalool solution and 0.45 mL of pyridine into the three-necked flask, and place them in an ice-salt bath environment with rapid stirring. Phosphate solution was added to the above reaction system at a rate of 1.0mL / min. After the dropwise addition was completed, the solution was continued for a rapid 45min. Dry over anhydrous sodium sulfate and use. Filter through a 0.22 μm microporous membrane, and concentrate by rotary evaporation at 30 °C for 30 min to obtain a pale yellow oily liquid linalyl bromide.
[0045] 2. Preparation of chitosan oligosaccharide-N-linalool copolymer
[0046]Dissolve 1g of chitosan oligosaccharide and 2mL of linalyl bromide with N,N-dimethylformamide respectively, add triethylamine as a catalyst, and slo...
Embodiment 2
[0056] Embodiment two: synthetic chitosan oligosaccharide-N-linalool copolymer
[0057] 1, the preparation of linalyl bromide
[0058] First dissolve 5mL of linalool and 2.5mL of phosphorus tribromide with 40mL of anhydrous ether, add the linalool solution and 0.5mL of pyridine into a three-necked flask, stir rapidly in an ice-salt bath environment, and then dissolve the three Phosphorus bromide solution was added to the above reaction system at a rate of 1.2mL / min. After the dropwise addition was completed, the solution was continued for 50 minutes, and then washed 3 times with 5% sodium bicarbonate solution, deionized water and saturated saline respectively. Add anhydrous sodium sulfate to dry, use. Filter through a 0.22 μm microporous membrane, and concentrate by rotary evaporation at 35° C. for 40 minutes to obtain light yellow oily liquid linalyl bromide.
[0059] 2. Oligochitosan-N-linalool copolymer
[0060] After 1g of oligochitosaccharide and 3mL of linalyl bromide...
Embodiment 3
[0065] Embodiment three: synthetic chitosan oligosaccharide-N-linalool copolymer
[0066] 1, the preparation of linalyl bromide
[0067] First dissolve 5mL of linalool and 3mL of phosphorus tribromide with 50mL of dehydrated ethanol respectively, add the linalool solution and 0.5mL of triethylamine in a three-neck flask, place it in an ice-salt bath environment and stir rapidly, and then Phosphorus tribromide solution was added to the above reaction system at a rate of 1.5mL / min. After the dropwise addition was completed, continue for 80 minutes, and then washed 3 times with 5% sodium bicarbonate solution, deionized water and saturated saline respectively. , dried by adding anhydrous sodium sulfate, filtered through a 0.45 μm microporous membrane, and concentrated by rotary evaporation at 45° C. for 50 min to obtain a light yellow oily liquid linalyl bromide.
[0068] 2. Preparation of oligochitosan-N-linalool copolymer
[0069] Dissolve 1g of oligochitosaccharide and 5mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of deacetylation | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com