Application of lignin flavanonol in preparation of antiviral drugs
A technology of dihydrogen and drugs, which is applied in the direction of antiviral agents, drug combinations, and pharmaceutical formulations, can solve the problems of not being effectively developed, and achieve the effects of inhibiting herpes simplex virus, high yield, and conducive to industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
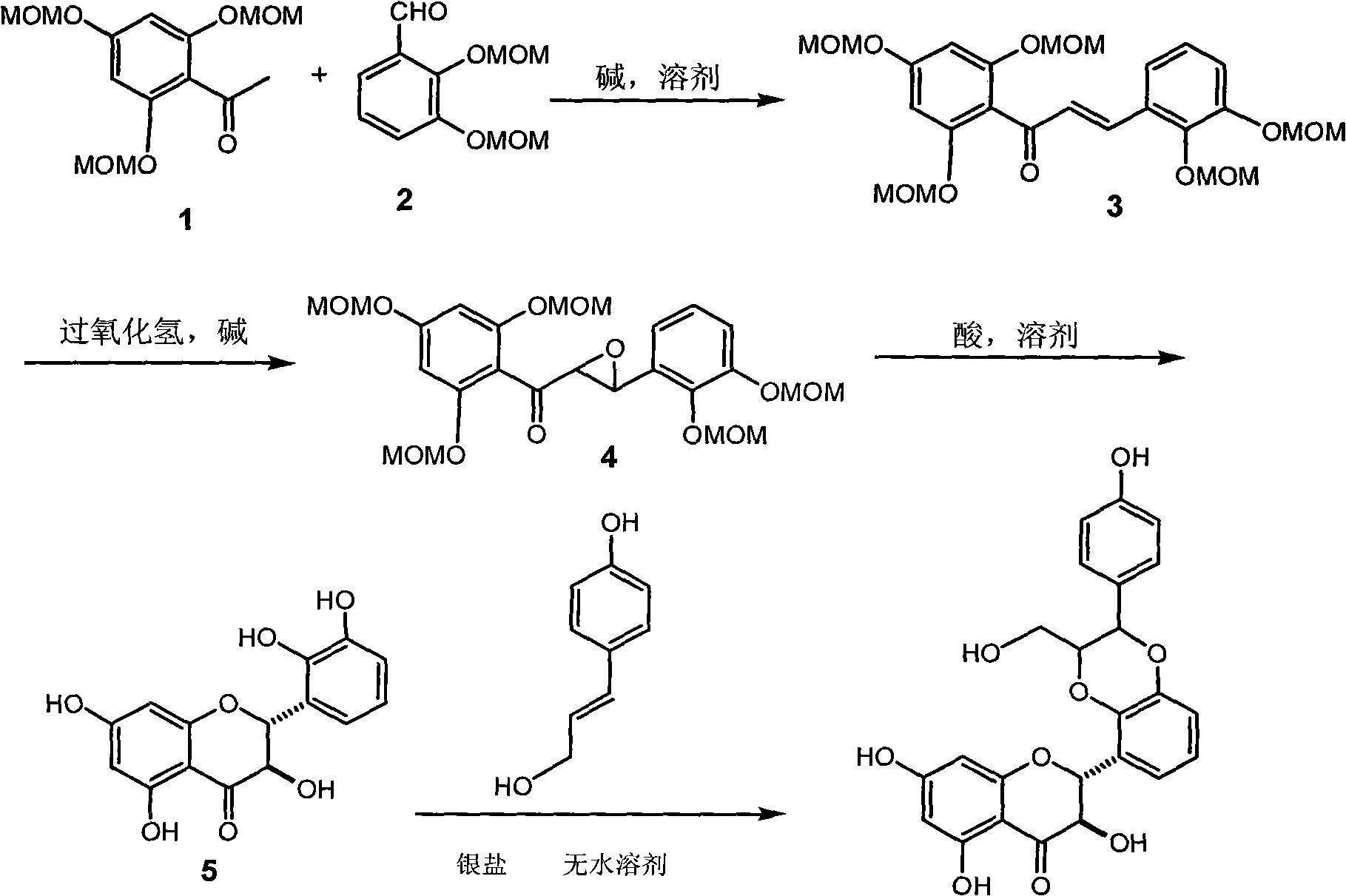
[0020] Example 1 : the preparation of intermediate epoxy chalcone (4)
[0021] 1.12, The preparation of 4,6-trimethoxymethoxyacetophenone (1):
[0022] 2.6 grams of sodium hydride in 40 milliliters of DMF was cooled in an ice-water bath, and a mixed solution of 5.6 grams of 2,4,6-trihydroxyacetophenone in 60 milliliters of benzene and 7.0 milliliters of DMF was added dropwise under nitrogen protection, and cooled in an ice bath 9.0 ml of chloromethyl ether solution was added dropwise, and stirred at room temperature for 24 hours. Pour into 100 ml of 10% sodium hydroxide aqueous solution, extract with ether 3 times, 50 ml each time, wash with saturated sodium bicarbonate, dry over anhydrous sodium sulfate, filter, concentrate, 40 g of 200-300 mesh silica gel column chromatography, petroleum Ether / ethyl acetate 4:1 was eluted to obtain 7.0 g of compound (1). Yellow oil; R f (Petroleum ether / ethyl acetate=3:1): 0.30; H NMR spectrum (400MHz, deuterated chloroform): δ2.52 (sing...
Embodiment 2
[0029] Example 2 : Preparation of (±)-2-(2,3-dihydroxyphenyl)2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one (5)
[0030] 1.0 grams of intermediate epoxy chalcone (4) was dissolved in 15 milliliters of methanol, and added under stirring to 10 milliliters of methanol solution in which 1.5 milliliters of concentrated hydrochloric acid was dissolved, and the temperature was raised to 60° C. for half an hour, then the heating was removed, and after cooling, the The solvent was removed by pressure evaporation, 50 ml of water was added to the residue, extracted with ethyl acetate (3 times, 20 ml each), the combined organic layers were washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure. After removing the solvent, the residue was subjected to 20 g of 200-300 mesh silica gel column chromatography, eluting with petroleum ether / ethyl acetate 3:1 to obtain 97 mg of (±)-2-(2,3-dihydroxyphenyl) 2 , 3-Dihydro-3,5,7-tr...
Embodiment 3
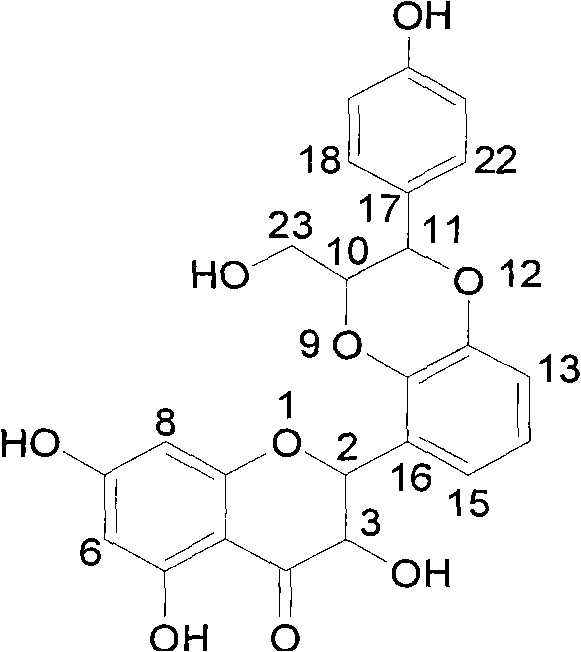
[0031] Example 3 : (±)-2-[2,3-dihydro-2-(4-hydroxyphenyl)-3-hydroxymethyl-1,4-benzodioxane-5]-2,3-dihydro - Preparation of 3,5,7-trihydroxy-4H-1-benzopyran-4-one
[0032] Put 0.22 grams of silver carbonate into the dry reaction bottle, add 20 milliliters of anhydrous benzene and 5 milliliters of anhydrous acetone, add dropwise 90 milligrams of (±)-2-(2,3-dihydroxyphenyl) 2,3 -dihydro-3,5,7-trihydroxy-4H-1-chromen-4-one (5) in dry benzene in 5 ml and 76 mg of p-hydroxycinnamyl alcohol in dry acetone in 3 ml, The reaction was incubated at 55°C for 20 hours. After cooling to room temperature, let it stand, filter off the insoluble matter, and concentrate the mother liquor under reduced pressure to obtain a yellow oil, which was subjected to 20 g of 200-300 mesh silica gel column chromatography and eluted with chloroform / methanol 10:1 to obtain 18 mg of the target compound.
[0033] (±) 2-[2,3-dihydro 2-(4-hydroxyphenyl)-3-hydroxymethyl-1,4 benzodioxane-5]-2,3-dihydro-3, 5,7-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com