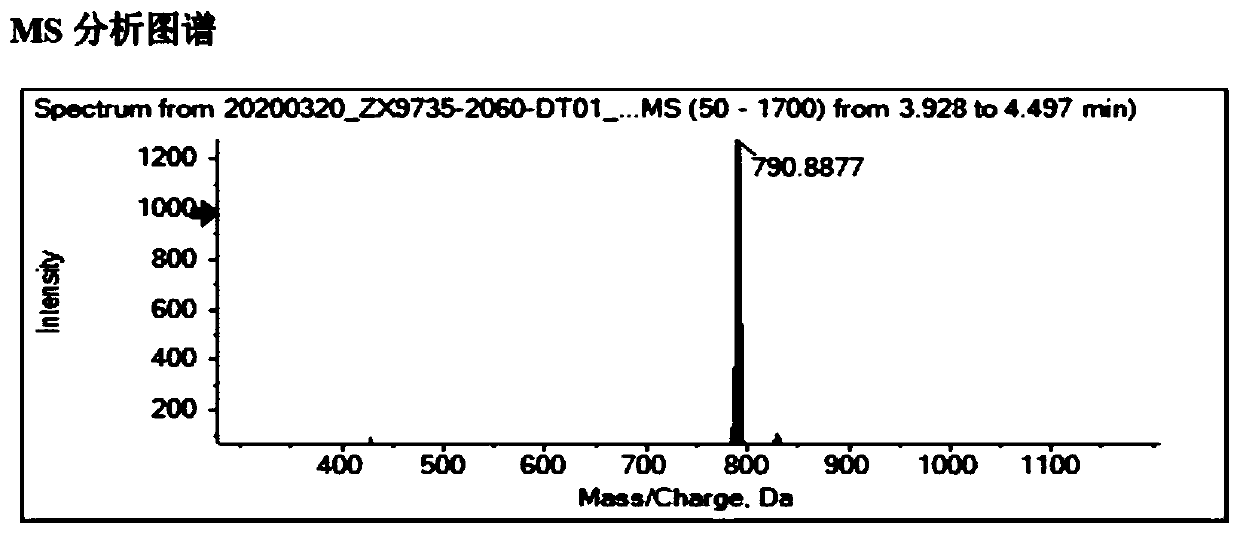
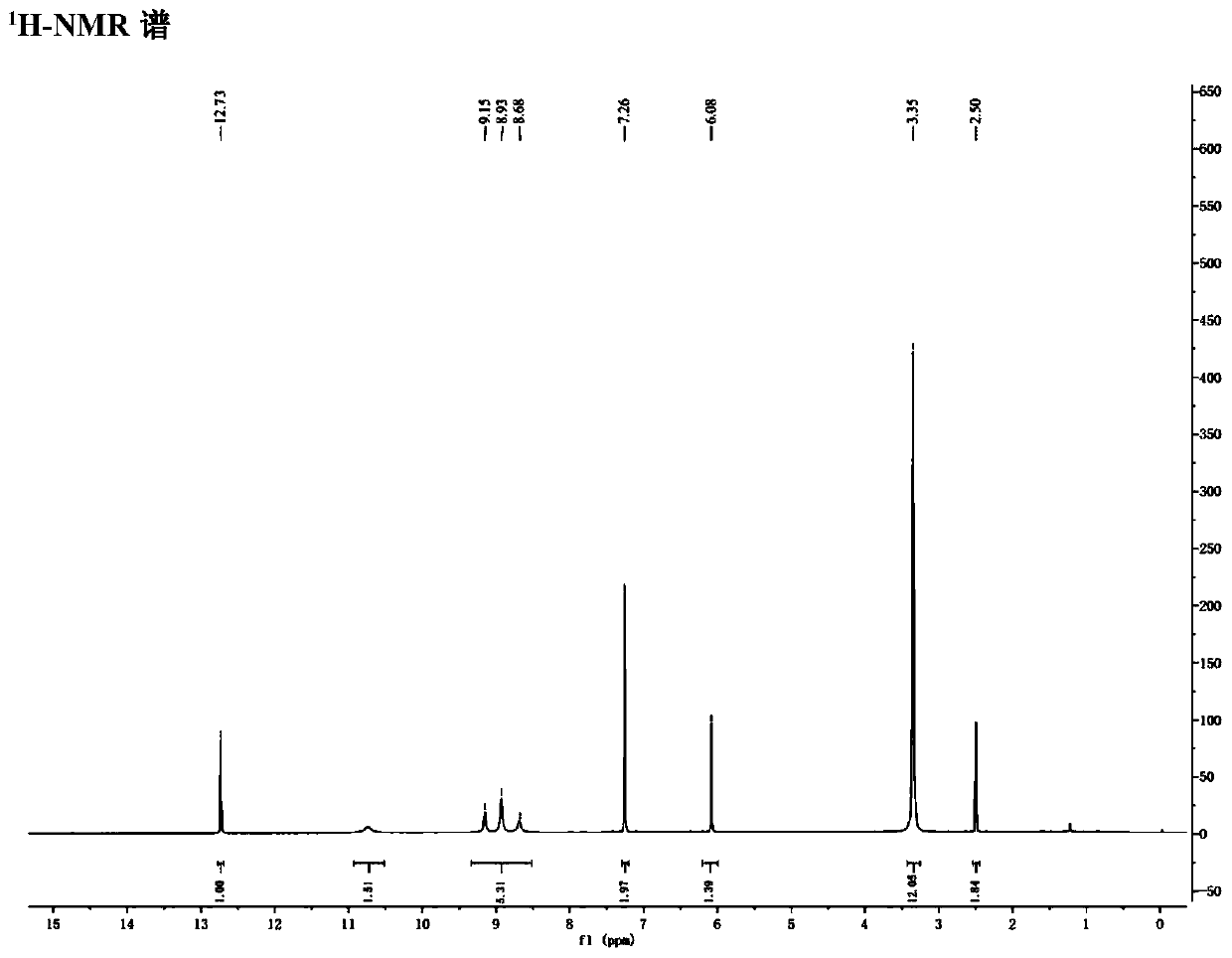
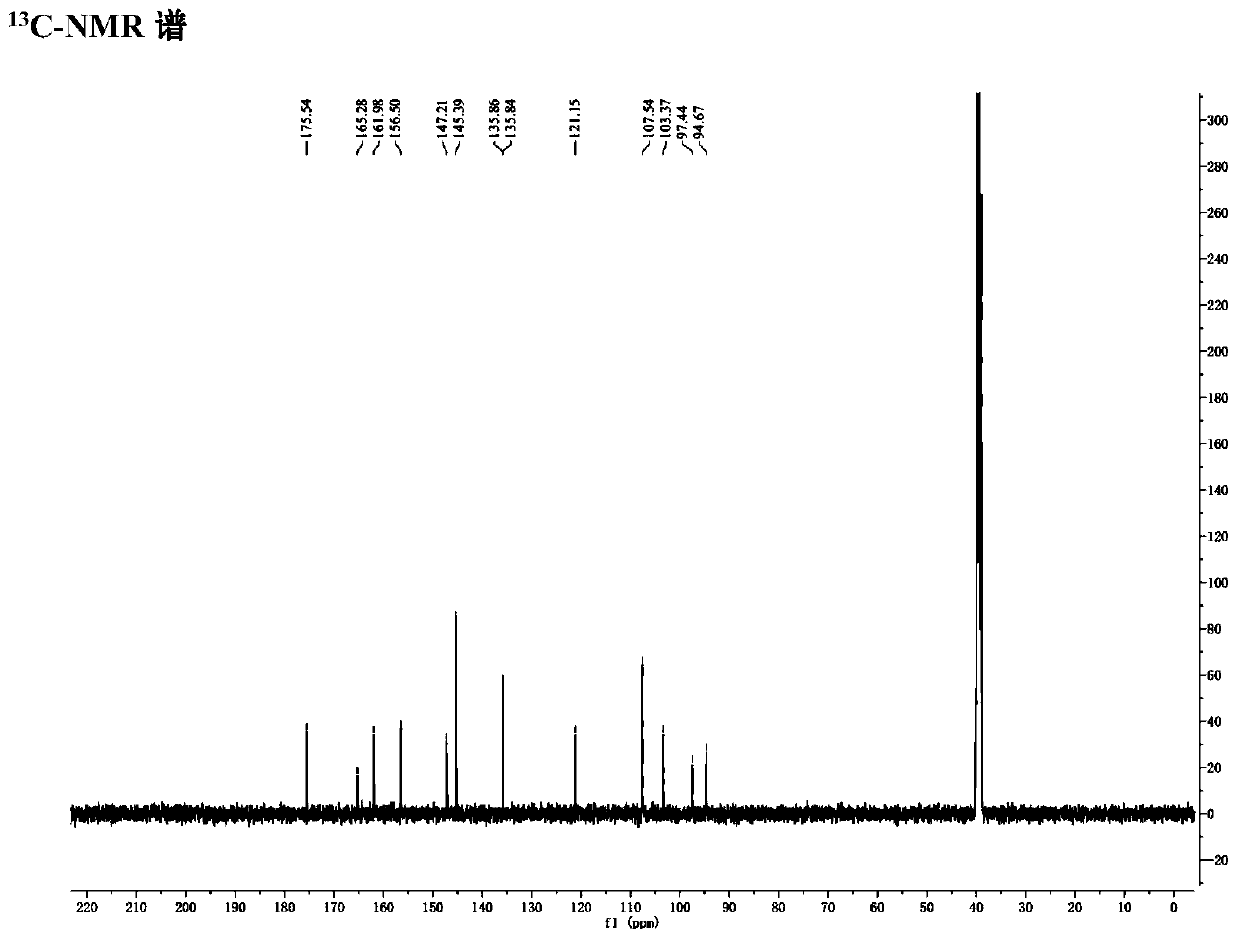
Process for preparing diselenide compound
A compound and diselenide technology, applied in the field of preparing diselenide compounds, can solve the problems of intense reaction of sodium borohydride, difficult to mass-produce, harsh reaction conditions, etc., and achieve easy control, suitability for large-scale industrial production, and stable reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In the present embodiment, the dihydroflavonol compound selects dihydromyricetin, and the structural formula is as follows:
[0026]
[0027] Add 22.5g of dihydromyricetin into a four-necked flask (500mL), add 200mL of water, heat to 80°C, keep for 45 minutes, add 2.81g of sodium hydroxide, control the temperature at 80°C for 10 minutes, then add 7.80 g selenium dioxide, controlled temperature and reacted for 70 minutes at 80° C., and the reaction was completed. Then, turn off the heating, let it cool down to room temperature naturally, adjust the pH value to 3-6.5 with hydrochloric acid, extract with methanol, separate by column chromatography, and freeze-dry to obtain the dimyricetin-based diselenide product with a yield of 8.63%.
Embodiment 2
[0029] In the present embodiment, the dihydroflavonol compound selects dihydromyricetin, and the structural formula is as follows:
[0030]
[0031] Add 15g of dihydromyricetin into a four-necked flask (specification: 500mL), add 200mL of water, heat to 90°C, keep for 45 minutes, add 0.75g of sodium hydroxide, control the temperature at 90°C for 60 minutes, then add 4.16 g of selenium dioxide, controlled temperature and reacted at 90° C. for 70 minutes, and the reaction was completed. Then, the heating was turned off, and after cooling down to room temperature naturally, the pH value was adjusted to 3-6.5 with hydrochloric acid, extracted with methanol, separated by column chromatography, and freeze-dried to obtain the product of dimyricetin-based diselenide with a yield of 10.26%.
Embodiment 3
[0033] In the present embodiment, the dihydroflavonol compound selects dihydromyricetin, and the structural formula is as follows:
[0034]
[0035] Add 22.5g of dihydromyricetin into a four-necked flask (specification: 500mL), add 200mL of water, heat to 100°C, keep for 45 minutes, add 2.53g of sodium hydroxide, control the temperature at 100°C for 40 minutes, then add 9.36g of selenium dioxide was reacted at 100° C. for 30 minutes under temperature control, and the reaction was completed. Then, the heating was turned off, and after cooling down to room temperature naturally, the pH was adjusted to 3-6.5 with hydrochloric acid, extracted with methanol, separated by column chromatography, and freeze-dried to obtain the product of dimyricetin-based diselenide with a yield of 9.86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com