Application of lignin flavanonol in preparation of antiviral drugs
A dihydrogen and drug technology, applied in the directions of antiviral agents, drug combinations, sexually transmitted diseases, etc., can solve the problems that have not been effectively developed, and achieve the effects of convenient source of raw materials, high yield and easy availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
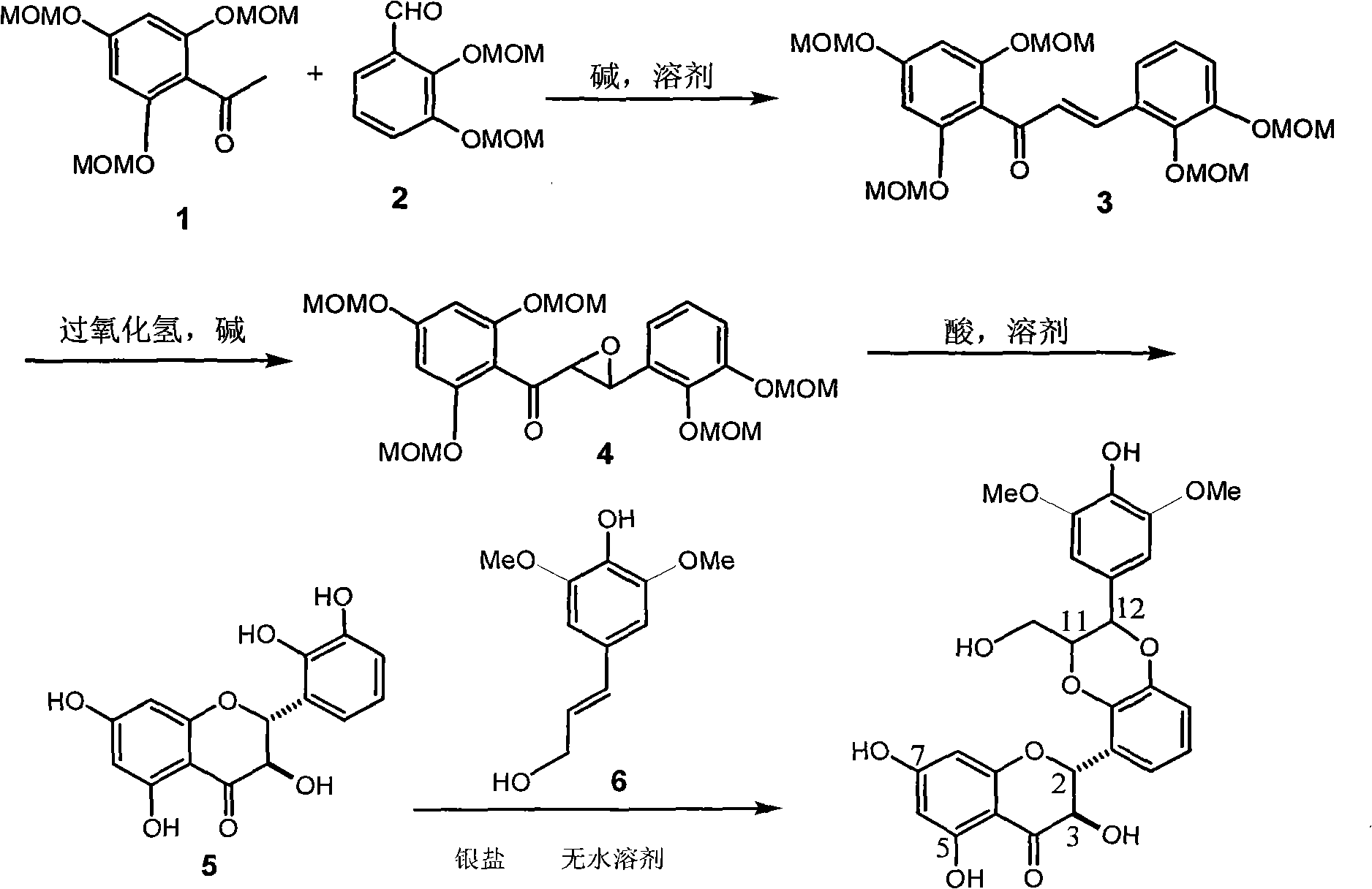
[0033] Example 1 : compound of formula (I) (±)-2-[2,3-dihydro-2-(3,5-dimethoxy-4-hydroxyphenyl)-3-hydroxymethyl-1,4benzo Preparation of dioxane-5]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one
[0034] 1.1 Preparation of starting material A-2,4,6-trimethoxymethoxyacetophenone (1):
[0035] Cool the solution of 2.6 g of sodium hydride in 40 ml of DMF in an ice-water bath, add dropwise a mixed solution of 5.6 g of 2,4,6-trihydroxyacetophenone in 60 ml of benzene and 7.0 ml of DMF under nitrogen protection, and cool in an ice-bath 9.0 ml of chloromethyl ether solution was added dropwise, and the mixture was stirred at room temperature for 24 hours. Pour into 100 ml of 10% aqueous sodium hydroxide solution, extract three times with 50 ml of ether, wash with saturated sodium bicarbonate, dry with anhydrous sodium sulfate, filter, concentrate, 40 g of 200-300 mesh silica gel column chromatography, petroleum Elution with ether / ethyl acetate 4:1 gave 7.0 g of starting material...
Embodiment 2
[0048] Example 2 : The cytotoxicity of the compound of formula (I) on Vero cells was determined by MTT method, and the inhibitory effect on HSV-1 was studied by trace cytopathic inhibition (CPE) method.
[0049] 2.1 Experimental purpose
[0050] Using HSV-1-infected Vero cells as a model, anti-HSV drugs were screened, wherein the sample was from the sample of the compound of formula (I) in Example 1.
[0051] 2.2 Materials
[0052] 2.2.1 Viruses and cells
[0053] 2.2.1.1 Virus: HSV-1 is provided by the Laboratory of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University;
[0054] 2.2.1.2 Cells: Vero cells were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
[0055] 2.3 Reagents
[0056] 2.3.1RPMI 1640 medium: product of Gibco;
[0057] 2.3.2L-Glutamine: AMRESCO products, subpackaged by Shanghai Sangon Bioengineering Technology Service Co., Ltd.;
[0058] 2.3.3MTT: AMRESCO products, subpackaged by Sha...
Embodiment 3
[0081] Example 3 : Inhibitory effect of compound of formula (I) on HSV-2 by microcytopathic inhibition (CPE) method
[0082] 3.1 Experimental purpose
[0083] Using HSV-2-infected Vero cells as a model, anti-HSV drugs were screened, wherein the samples were from the sample of the compound of formula (I) prepared in Example 1.
[0084] 3.2 Materials
[0085] 3.2.1 Viruses and cells
[0086] 3.2.1.1 Virus: HSV-2 is provided by the Institute of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University;
[0087] 3.2.1.2 Cells: Vero cells were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
[0088] 3.2.2 Reagents
[0089] 3.2.2.1RPMI 1640 medium: product of Gibco;
[0090] 3.2.2.2L-Glutamine: AMRESCO product, subpackaged by Shanghai Sangon Bioengineering Technology Service Co., Ltd.;
[0091] 3.2.2.3MTT: AMRESCO products, subpackaged by Shanghai Sangon Bioengineering Technology Service Co., Ltd.;
[0092] 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com