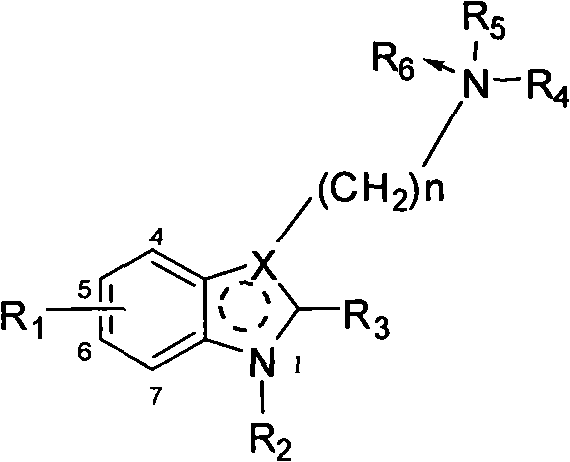
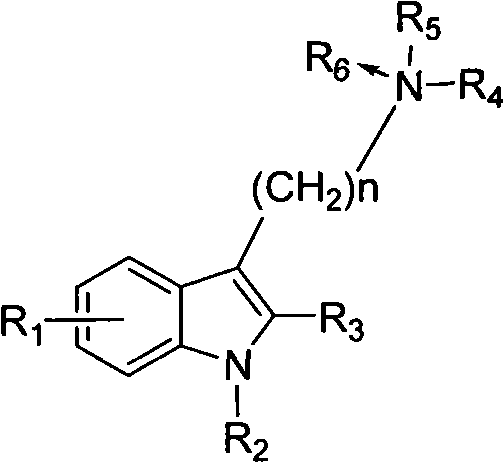
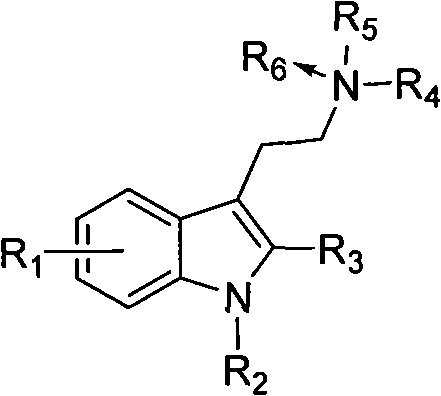
Benzohetercyclic compound as well as preparation method and applications thereof
A technology of compounds and heterocycles, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1 Extraction of tryptamine alkaloids from plants
[0107] The roots and stems (20kg) of Jiangxi Rutaceae Evodia rutaecarpa were extracted three times by percolation at room temperature with 95% ethanol, the extracts were combined and concentrated under reduced pressure to obtain ethanol extract, and the ethanol extract was suspended in water (3L) Sequentially extract with petroleum ether, chloroform, ethyl acetate and n-butanol (each 3L×3); 78g of the n-butanol extraction part is acidified with 2% hydrochloric acid, and the precipitate is removed by filtration, and the acidic water layer is alkalized with concentrated ammonia water, adjusted The pH is about 8-9, and then extracted with n-butanol until there is no alkaloid reaction in the n-butanol layer, and 6.7 g of total alkaloids are obtained; the sample is mixed with silica gel, and the gradient elution is performed by normal phase silica gel column chromatography (CHCl 3 -MeOH 50:1, 20:1, 12:1, 8:1, 5:1, 3:...
Embodiment 25
[0110] Example 25-Hydroxy-N 12 - Oxy-N,N-Dimethyltryptamine
[0111]
[0112] Reagents and conditions: (a) NaOMe, MeOH, formaldehyde solution (38%), NaCNBH 3 , AcOH, room temperature, 2h; (b) MCPBA (50%), CHCl 3 , 0°C, 5min.
[0113] Specific test operation:
[0114] (a) Under an argon atmosphere, dissolve 200mg of 5-hydroxytryptamine hydrochloride (0.94mmol) in 10ml of anhydrous methanol, cool in an ice bath, and add fresh sodium methoxide (20mgNa dissolved in 1ml of anhydrous methanol) while stirring. Adjust pH8-9, then add 0.22ml acetic acid (3.76mmol) to adjust pH5-6, then add 102mg sodium cyanoborohydride (1.88mmol), finally add dropwise 0.18ml (2.26mmol) of 38% aqueous formaldehyde solution, the reaction temperature rises to At room temperature, continue to stir for 2h (TLC detects the reaction process), after the reaction is complete, add 2mol / L Na dropwise 2 CO 3 Adjust the pH of the solution to about 8-9 to terminate the reaction, filter, and concentrate the ...
Embodiment 3
[0130] Example 3N-Methyl-N 12 - Amyltryptamine
[0131]
[0132] Reagents and conditions: (a) MeOH, Isovaleraldehyde, NaCNBH 3 , AcOH, 0°C, 1h; (b) formaldehyde solution (38%) (excess), room temperature, 8h.
[0133] Specific test operation:
[0134] (a) Dissolve 100mg of tryptamine (0.62mmol) in 5ml of methanol, cool in an ice bath, add 0.14ml of acetic acid (2.48mmol) under stirring to adjust the pH to 5-6, then add 78mg of NaCNBH 3 (1.24mmol), finally dropwise added 0.067ml (0.62mmol) of isovaleraldehyde (0.62mmol) methanol (20ml) solution under ice bath cooling, the dropwise addition time was greater than 1h, the reaction temperature was kept at 0°C and continued to stir for 1h, and TLC detected that the reaction was complete .
[0135] (b) The reaction solution of the above (a) step is not treated, and the stirring is continued at room temperature, 0.06ml (0.75mmol) of 38% formaldehyde solution is added, and the stirring is continued for 1.5h (TLC detection reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com