HBV nucleic acid quantitative detection system based on micro-droplet digital PCR technology
A technology of nucleic acid quantification and detection system, applied in the field of digital PCR, which can solve the problems of difficult judgment of results and insufficient accuracy of qPCR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Quantitative detection of HBV DNA by micro-droplet digital PCR
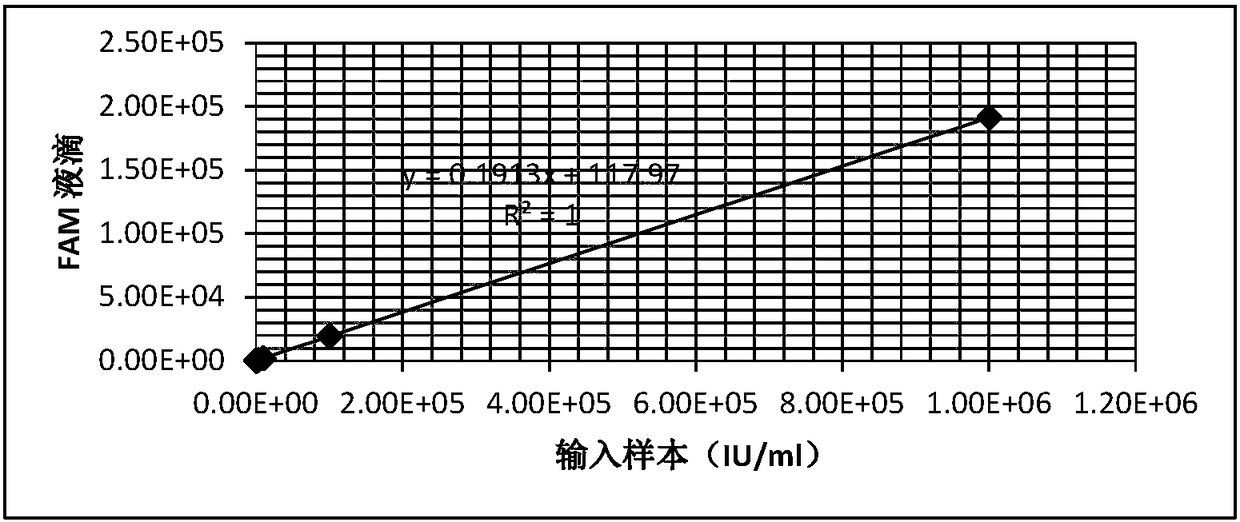
[0026] Sample selection: Use high-value clinical samples (quantified by methods traceable to national reference materials), and perform serial dilution with HBV-negative plasma to obtain 10 6 、10 5 、10 4 、10 3 , 100 and 10IU / ml concentrations were tested, and the plasma volume was 500μl.
[0027] DNA extraction: nucleic acid extraction was performed using a virus nucleic acid extraction system (Thermo Fisher, 37011D), and the operation steps were performed according to the system manual. Extracted nucleic acids should be stored at -20°C and long-term stored at -80°C.
[0028] PCR reaction system preparation: the system includes 1×PCR reaction buffer (containing 50mmol / L Tris.HCl (PH8.3), 10mmol / L potassium chloride, 5mmol / L ammonium sulfate, 2.5mmol / L magnesium chloride); Glycoside triphosphate dATP, dCTP, dGTP and dUTP, the final concentration is 200nmol / L, and the final concentration of dU...
Embodiment 2
[0065] Embodiment 2: PCR system anti-pollution ability
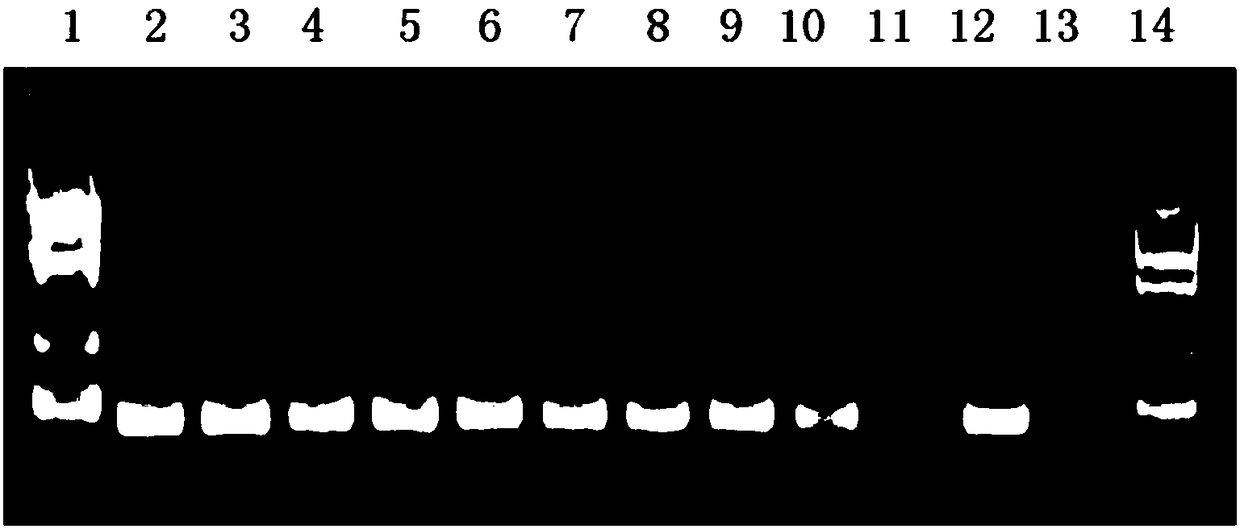
[0066] After the dU-containing PCR product was purified, it was quantified by a UV spectrophotometer and the copy number was calculated, and the dilution was quantified to about 1×10 10 copies / μl, then serially diluted 10 times to 10 -10 dilution. Take dU-containing PCR products of various dilutions as templates for simulating pollution sources, and use this system for PCR amplification. The amplified products were detected by agarose gel electrophoresis, and the experimental results were as follows: figure 2 shown.
[0067] The experimental results show that 10 3 copy / reaction (corresponding to 10 -7 dilution) and below simulated pollution sources, no amplified product bands were seen; while for the control PCR amplification system without dUTP and UNG, it was as low as 10 1 The simulated pollution source of the copy / reaction can still see clear amplification product bands, and the experimental results are as fol...
Embodiment 3
[0068] Embodiment 3: the detection of clinical sample
[0069] Collect clinical HBV positive samples (with high, medium and low concentration distribution) and HBV negative samples, a total of 130 samples (30 HBV positive samples, 100 HBV negative samples), carry out micro-droplet digital PCR reaction, and compare with the current HBV The gold standard in the DNA quantification industry - Roche Cobas Amliprep / Cobas TaqMan products are compared, and the data are statistically analyzed. The detection sensitivity of the HBV quantitative detection system based on micro-droplet digital PCR technology in the present invention is 100% (30 / 30), The specificity was 100% (100 / 100). The quantitative results are highly consistent with Roche Cobas Amliprep / Cobas TaqMan products. This shows that the detection system based on the digital PCR system of the present invention has high consistency with Roche products, and can realize accurate absolute quantification of HBV DNA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com