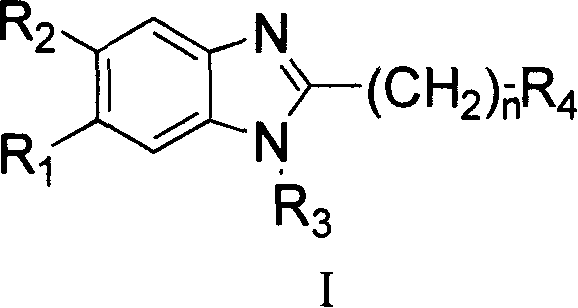
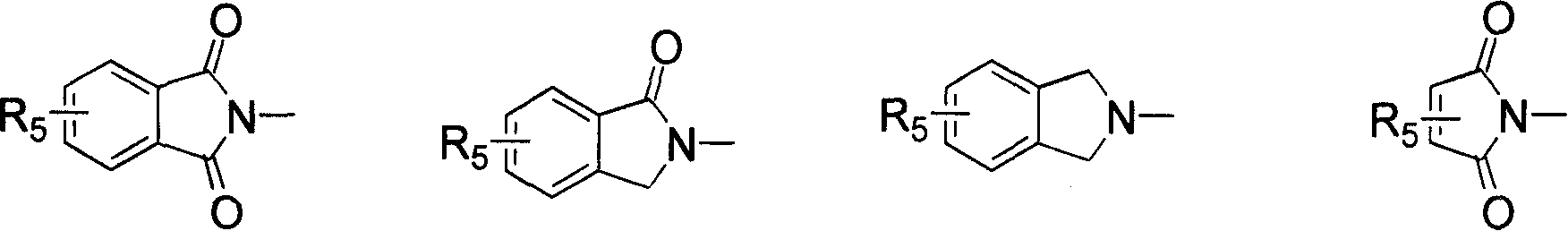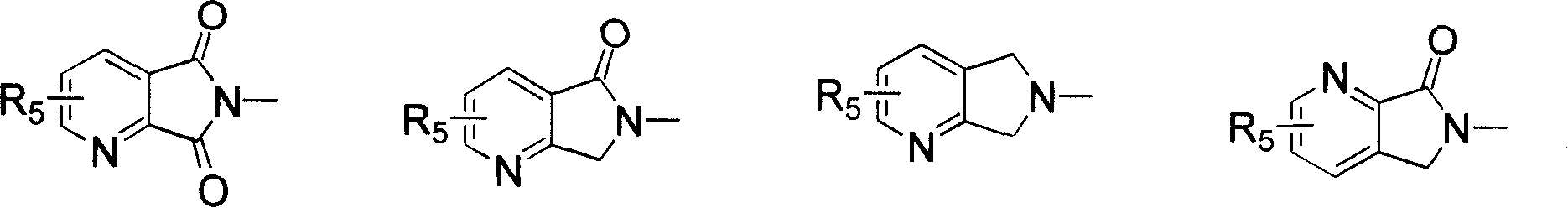Benzimidazole compounds, its prepn. and uses
A technology of benzimidazoles and compounds, applied in the field of benzimidazoles compounds, can solve the problems of inability to be universally vaccinated due to immune effects, treatment of hepatitis B patients, serious side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0591] Example 1 Synthesis of 2-[2-(1H-benzimidazol-2-yl)-ethyl]-isoindole-1,3-dione (BMHPY)
[0592] Mix 11g (0.05mole) 3-phthalimidopropionic acid and 5.4g (0.05mole) o-phenylenediamine evenly, add 50g polyphosphoric acid (PPA), react at 175-180°C for 6 hours, cool Then pour into cold water, filter, and the filtrate is neutralized to alkaline (pH>12) with concentrated ammonia water, and the product is filtered out, which can be directly used in the next step without purification. A total of 12.4 g was obtained after drying, and the yield was 85.1%. 1 H-NMR (400Hz, DMSO-d 6 )δ12.2-12.4 (br s, 1H), 7.82-7.87 (m, 4H), 7.42-7.46 (m, 2H), 7.10-7.13 (m, 2H), 4.01-4.05 (t, 2H, J= 7.2Hz), 3.14-3.18(t, 2H, J=7.2Hz); MS (M + )291.
Embodiment 2
[0593] Example 2 Synthesis of 2-[2-(5,6-dichloro-1H-benzimidazol-2-yl)-ethyl]-isoindole-1,3-dione (BMCPY)
[0594] 3-phthalimidopropionic acid and equivalent 4,5-dichloro-o-phenylenediamine were prepared according to the method of BMHPY to obtain 14.8 g of the product with a yield of 82.2%.
[0595] 1 H-NMR (400Hz, DMSO-d 6 )δ12.5-12.7 (br s, 1H), 7.81-7.86 (br s, 4H), 7.71 (s, 2H), 4.00-4.03 (t, 2H, J=7.2Hz), 3.15-3.18 (t, 2H, J=7.0Hz);
[0596] MS (M + )359.
Embodiment 3
[0597] Example 3 Synthesis of 2-[2-(5,6-difluoro-1H-benzimidazol-2-yl)-ethyl]-isoindole-1,3-dione (BMFPY)
[0598] 3-phthalimidopropionic acid and equivalent 4,5-difluoro-o-phenylenediamine were prepared according to the method of BMHPY to obtain 13.6 g of the product with a yield of 82.9%.
[0599] 1 H-NMR (400Hz, DMSO-d 6 )δ12.4-12.6 (br s, 1H), 7.82-7.86 (br s, 4H), 7.46-7.50 (m, 2H), 3.98-4.02 (t, 2H, J=7.6Hz), 3.13-3.17 ( t, 2H, J=7.6Hz);
[0600] MS (M + )327.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com