Patents
Literature
103 results about "Respiratory complex II" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
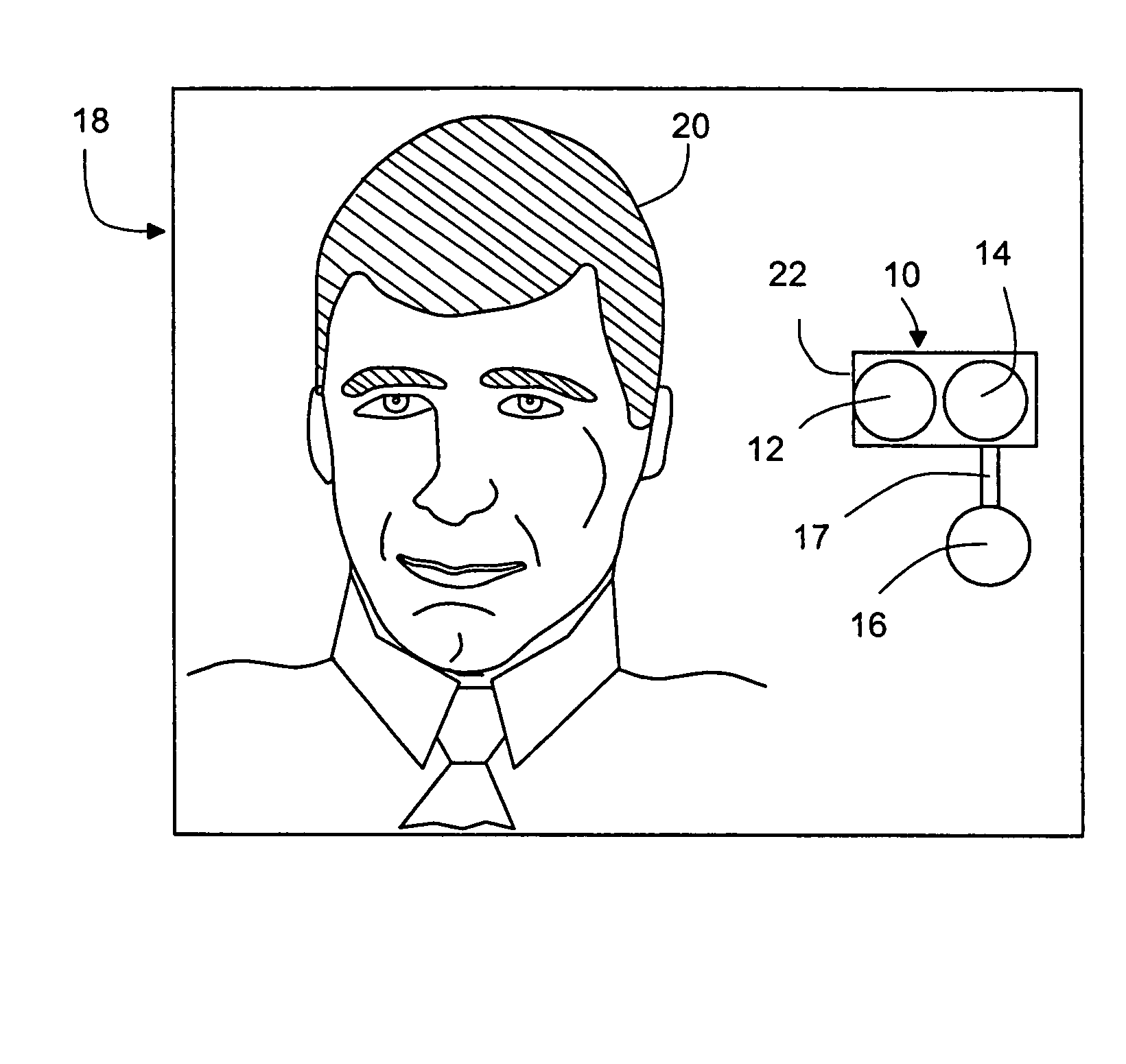
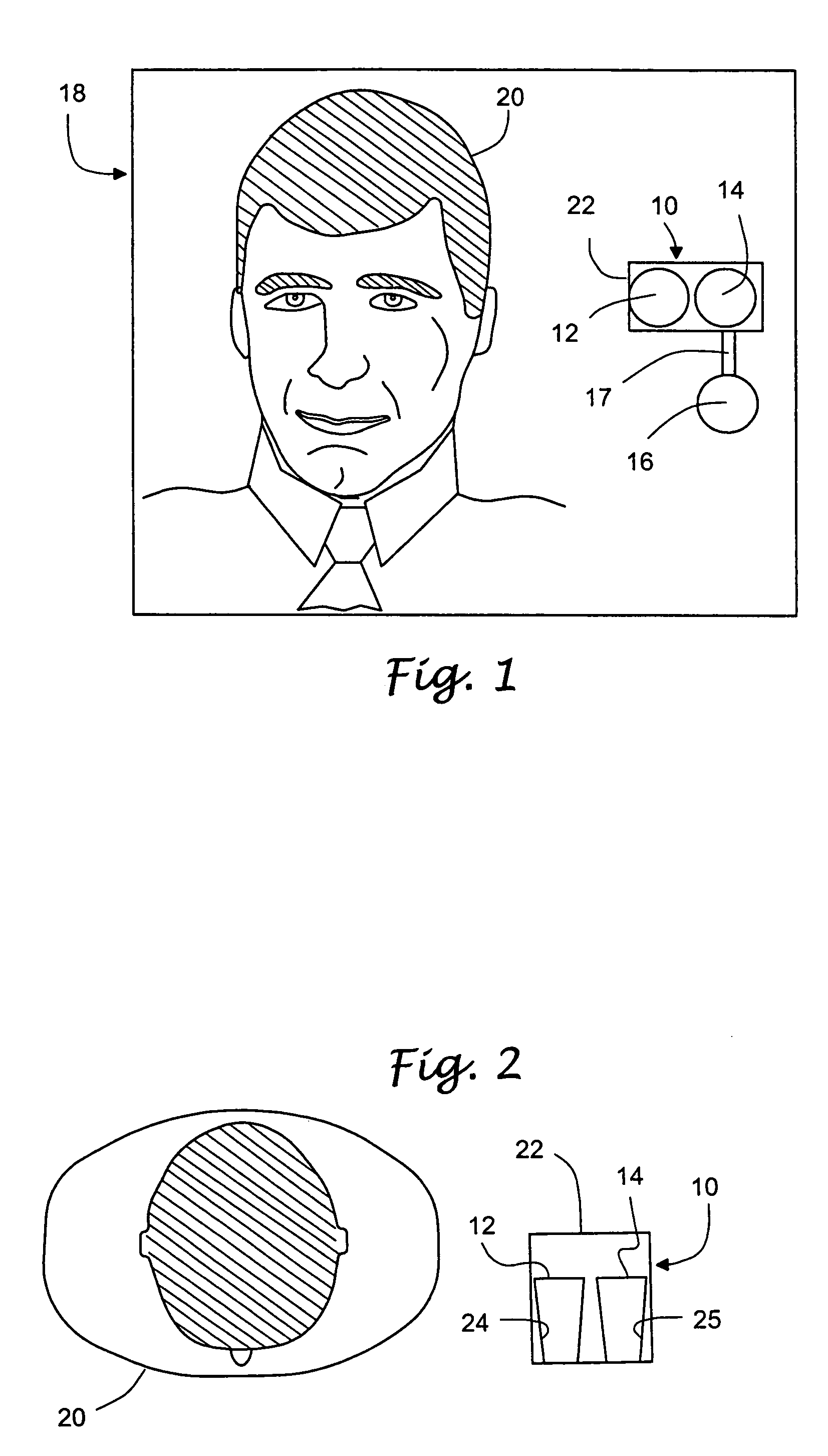
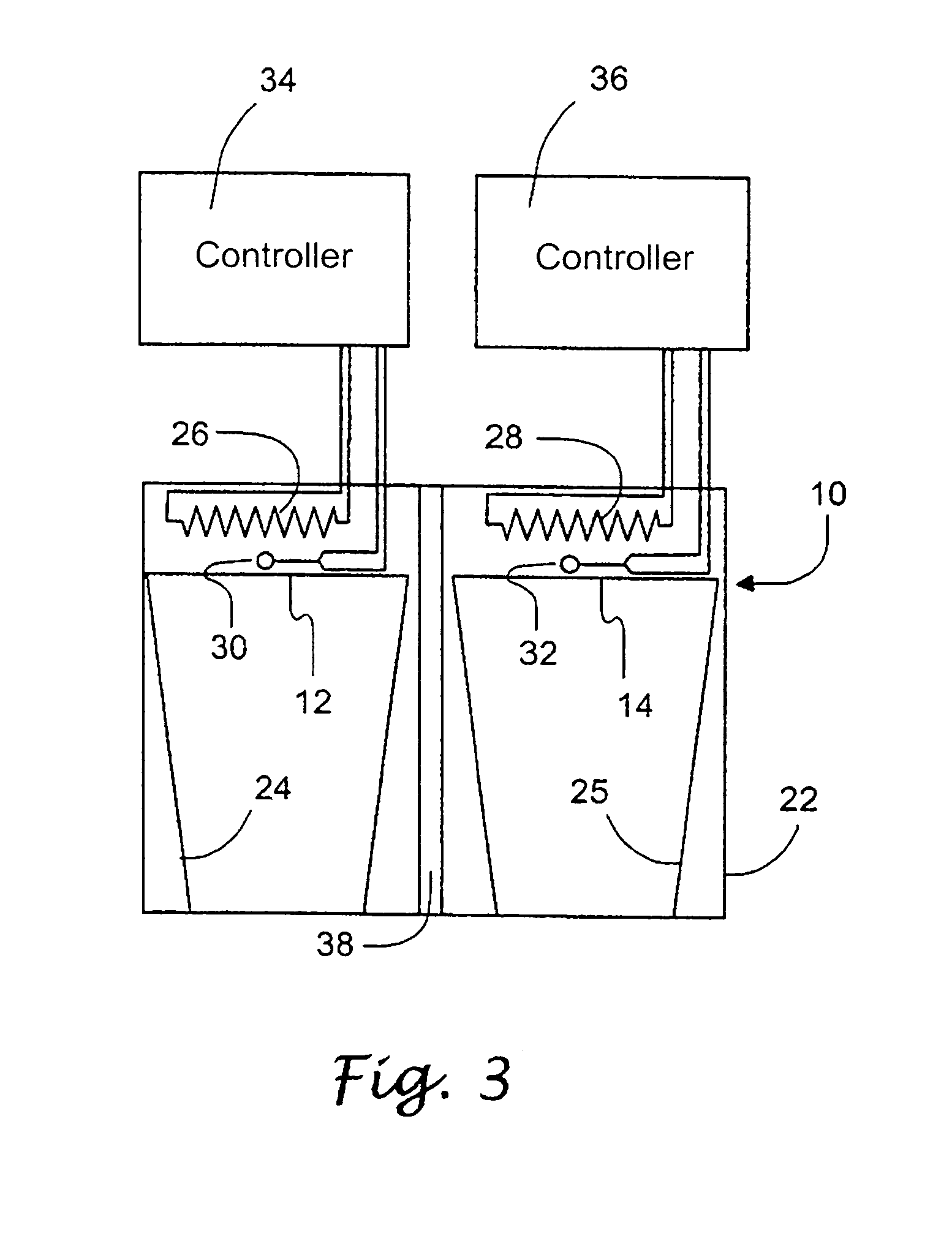
Methods and apparatus for a remote, noninvasive technique to detect core body temperature in a subject via thermal imaging
ActiveUS7340293B2Accurate of core body temperatureConvenience and securitySensing radiation from moving bodiesDiagnostic recording/measuringDiseaseEngineering
An approach to noninvasively, remotely and accurately detect core body temperature in a warm-blooded subject, human or animal, via thermal imaging. Preferred features such as the use of in-frame temperature references, specific anatomical target regions and a physiological heat transfer model help the present invention to overcome pitfalls inherent with existing thermal imaging techniques applied to physiological screening applications. This invention provides the ability to noninvasively, remotely and rapidly screen for diseases or conditions that are characterized by changes in core body temperature. One human application of this invention is the remote screening for severe acute respiratory syndrome (SARS), since fever is a common, early symptom. Other diseases and conditions that affect the core body temperature of humans or animals may also be noninvasively and remotely detected with this invention.
Owner:CARDIOWAVE
Diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
ActiveUS7267942B2Sugar derivativesMicrobiological testing/measurementPcr assaySevere acute respiratory syndrome
The present invention relates to a diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a real-time quantitative PCR assay for the detection of hSARS virus using reverse transcription and polymerase chain reaction. Specifically, the quantitative assay is a TaqMan® assay using the primers and probes constructed based on the genome of the hSARS virus. The invention further relates to a diagnostic kit that comprises nucleic acid molecules for the detection of the hSARS virus.
Owner:HONG KONG THE UNIV OF +1
Methods and apparatus for a remote, noninvasive technique to detect core body temperature in a subject via thermal imaging
InactiveUS20080154138A1Convenience and securityGuarantee economic securitySensing radiation from moving bodiesDiagnostic recording/measuringDiseaseEngineering
Owner:MCQUILKIN GARY L
Compositions and methods for detecting severe acute respiratory syndrome coronavirus
InactiveUS20050095582A1Lower Level RequirementsReduce rateSsRNA viruses positive-senseMicrobiological testing/measurementSARS coronavirusSevere acute respiratory syndrome coronavirus
The invention provides compositions and methods for detecting the presence of SARS-coronavirus, for screening anti-SARS coronavirus agents and vaccines, and for reducing infection with plus-strand RNA viruses such as SARS-coronavirus.
Owner:DIAGNOSTIC HYBRIDS +1
High-throughput diagnostic assay for the human virus causing severe acute respiratory syndrome (SARS)
InactiveUS7547512B2Rapid and reliable diagnostic assayHigh detection sensitivitySugar derivativesMicrobiological testing/measurementPresent methodSevere acute respiratory syndrome
The present invention relates to a high-throughput diagnostic assay for the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). In particular, the invention relates to a high-throughput reverse transcription-PCR diagnostic test for SARS associated coronavirus (SARS-CoV). The present assay is a rapid, reliable assay which can be used for diagnosis and monitoring the spread of SARS and is based on the nucleotide sequences of the N (nucleocapsid)-gene of the hSARS virus. The present method eliminates false negative results and provides increased sensitivity for the assay. The invention also discloses the S (spike)-gene of the hSARS virus. The invention further relates to the deduced amino acid sequences of the N-gene and S-gene products of the hSARS virus and to the use of the N-gene and S-gene products in diagnostic methods. The invention further encompasses diagnostic assays and kits comprising antibodies generated against the N-gene or S-gene product.
Owner:VERSITECH LTD
Attenuated Live Vaccine for Prevention of Porcine Reproductive and Respiratory Syndrome
ActiveUS20120189655A1Organic active ingredientsSsRNA viruses positive-senseBiologyAttenuated Live Vaccine
The present disclosure provides an attenuated live vaccine strain and the formulations thereof, for preventing pigs from infection of porcine reproductive and respiratory syndrome (PRRS). The preparation methods for the vaccines and the formulations are also provided. The attenuated live vaccine strain provided herein offers significant immunological protection to pigs against PRRS. The vaccine formulations of the present disclosure also have advantages in long shelf lives as well as good stability during storage.
Owner:WU HUA
Porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triple virus-like particle vaccine and its preparation method
The purpose of the invention is to disclose a porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triplex virus-like particle vaccine and its preparation method. The triple virus-like particle vaccine (Triple VLP vaccine) of the invention contains VLP which is composed of PCV-2 major structural protein CAP protein, PPV VP2 protein epitope and PRRSV Gp5 protein epitope. It is proved by experiment that the vaccine can stimulate good double cellular and humoral immune response. It is shown by pharmacodynamic test that after immunization of different animal groups, the vaccine of injection, nose drops and water forms prepared by VLP antigen formed by the method with or without adjuvants can safely and effectively prevent the infection of PCV-2, PPV and PRRSV. The invention provides an ideal vaccine for the security of sows, piglets and fattening pigs to effectively prevent mixed infection of PCV-2, PPV and PRRSV.
Owner:CHONGQING UNIV
Porcine reproductive and respiratory syndrome virus and methods of use
InactiveUS7041443B2Effective treatmentSsRNA viruses positive-senseVirus peptidesRespiratory syndrome virusImmunogenicity
The present invention provides isolated European-like porcine reproductive and respiratory syndrome viruses, polynucleotides, and polypeptides. The present invention also provides methods for making antibodies to the viruses and polypeptides, methods for detecting porcine reproductive and respiratory syndrome viruses, immunogenic compositions, and methods for treating a porcine subject at risk of infection by, or displaying symptoms of, a porcine reproductive and respiratory syndrome virus infection.
Owner:RGT UNIV OF MINNESOTA
Uses of interferons with altered spatial structure
InactiveUS20090220456A1Preventing and treating Severe AcutePeptide/protein ingredientsMicroorganismsSpatial structureInterferon alpha
This invention provides a method for preventing or treating Severe Acute Respiratory Syndrome in a subject comprising administering to the subject an effective amount of recombinant super-compound interferon or a functional equivalent thereof. This invention provides a method for inhibiting the causative agent of Severe Acute Respiratory Syndrome comprising contacting the agent with an effective amount of super-compound interferon or its equivalent.
Owner:WEI GUANGWEN
Application of l-sophocarpine on preparing medication for curing disease caused by coronavirus
InactiveCN1600308ARich sources of medicineSmall side effectsOrganic active ingredientsPharmaceutical delivery mechanismDiseaseMortality rate
An application of sophocarpine in preparing the medicine for treating the medicines caused by coronavirus and the process for preparing the medicine from sophocarpine to treat SARS are disclosed.
Owner:RENJI HOSPITAL ATTACHED TO SHANGHAI NO 2 MEDICALUNIV +1
Permissive cells and uses thereof
ActiveUS20100158947A1Compound screeningSsRNA viruses positive-senseVaccine ProductionFamily Arteriviridae
Owner:UNIV GENT ENGLISH TRANSLATION BEING GHENT UNIV
Novel human virus causing severe acute respiratory syndrome (SARS) and uses thereof
The present invention relates to an isolated novel virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). The hSARS virus is identified to be morphologically and phylogenetically similar to known member of Coronaviridae. The present invention provides the complete genomic sequence of the hSARS virus. Furthermore, the invention provides the nucleic acids and peptides encoded by and / or derived from the hSARS virus and their use in diagnostic methods and therapeutic methods, including vaccines. In addition, the invention provides chimeric or recombinant viruses encoded by said nucleotide sequences and antibodies immunospecific to the polypeptides encoded by the nucleotide sequences.
Owner:VERSITECH LTD
Attenuated live vaccine for prevention of porcine reproductive and respiratory syndrome
ActiveUS8728487B2Organic active ingredientsSsRNA viruses positive-senseBiologyAttenuated Live Vaccine
The present disclosure provides an attenuated live vaccine strain and the formulations thereof, for preventing pigs from infection of porcine reproductive and respiratory syndrome (PRRS). The preparation methods for the vaccines and the formulations are also provided. The attenuated live vaccine strain provided herein offers significant immunological protection to pigs against PRRS. The vaccine formulations of the present disclosure also have advantages in long shelf lives as well as good stability during storage.
Owner:WU HUA
Isolation and characterization of the precursor virus of human sars virus: sars-associated corona virus-like virus
ActiveUS20080069839A1SsRNA viruses positive-senseSugar derivativesViunalikevirusSevere acute respiratory syndrome
The present invention relates to isolation and characterization of a class of isolated novel viruses which is the precursor of the virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). The precursor virus which is a SARS coronavirus-like virus (“SCoV-like virus”) is identified to be morphologically and phylogenetically similar to hSARS virus. The present invention relates to a nucleotide sequence comprising the genomic sequence of the SCoV-like virus. The invention further relates to nucleotide sequences comprising a portion of the genomic sequence of the SCoV-like virus. The invention also relates to the deduced amino acid sequences of the SCoV-like virus. The invention further relates to the nucleic acids and peptides encoded by and / or derived from these sequences and their use in diagnostic methods and therapeutic methods. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences and antibodies directed against polypeptides encoded by the nucleotide sequences. Furthermore, the invention relates to vaccine preparations comprising the SCoV-like virus, including recombinant and chimeric forms of said virus.
Owner:THE UNIVERSITY OF HONG KONG
Compositions and methods for detecting severe acute respiratory syndrome coronavirus
InactiveUS7129042B2Improve productivityHigh sensitivitySsRNA viruses positive-senseMicrobiological testing/measurementSARS coronavirusSevere acute respiratory syndrome coronavirus
The invention provides compositions and methods for detecting the presence of SARS-coronavirus, for screening anti-SARS coronavirus agents and vaccines, and for reducing infection with plus-strand RNA viruses such as SARS-coronavirus.
Owner:DIAGNOSTIC HYBRIDS +1
Combined vaccines for prevention of porcine virus infections
ActiveUS20140093535A1SsRNA viruses positive-senseViral antigen ingredientsProtection sexProtective immunity
The present disclosure provides vaccine compositions comprising a PRRSV vaccine and a second porcine vaccine, which are substantially free from immuno-inhibition against each other. The second porcine virus vaccine can be CSFV and / or PRV. The preparation methods for the vaccines and the formulations are also provided. The vaccine compositions provided herein confer protective immunity to pigs against porcine reproductive and respiratory syndrome, classical swine fever, and / or pseudorabies.
Owner:SINOVET JIANGSU BIOPHARM CO LTD
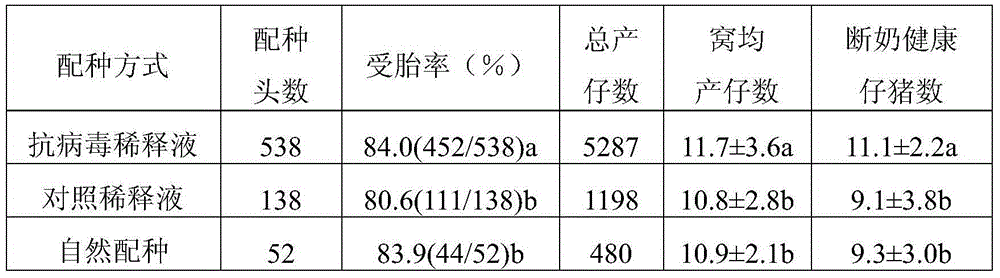
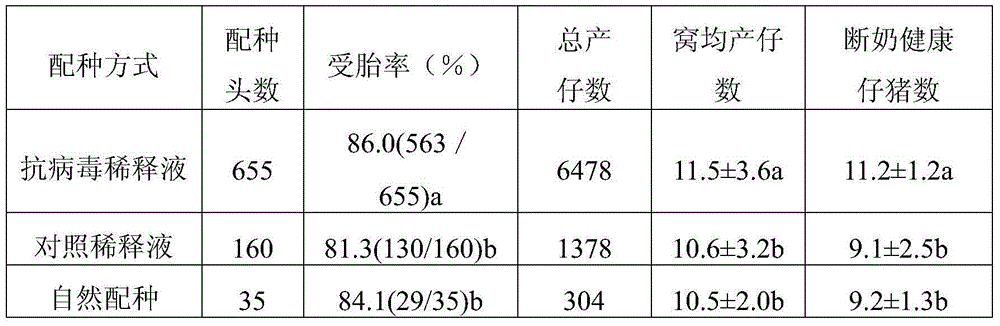
Prescription composition of pig antiviral semen diluent
InactiveCN105010304AObvious killObvious inhibitoryBiocideDead animal preservationSodium bicarbonateVitamin C
The invention discloses a prescription composition of a pig antiviral semen diluent. Based on every 1000ml of double distilled water, the pig antiviral semen diluent contains 15.50g of glucose, 11.65g of trisodium citrate, 2.35g of EDTA sodium salt, 1.75g of sodium bicarbonate, 1.00g of polyvinyl alcohol, 5.50g of tricarboxymethyl amino methane, 4.10g of citric acid, 0.07g of cysteine, 1.00g of vitamin C, 0.10g of enrofloxacin and 50ml of an antiviral agent. According to the embodiment of the invention, the diluent of the prescription composition of the pig antiviral semen diluent has obvious killing, inhibition and obstructing effects on pig reproduction and respiration syndrome virus (PRRSV) and is capable of obviously improving the survival rate and preservation time of semen during in vitro preservation and the litter size of a mated sow.
Owner:马乃祥
Triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and preparation method and application thereof
InactiveCN104745731AMicrobiological testing/measurementMicroorganism based processesPositive controlAfrican swine fever
The invention discloses a triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and a preparation method and application thereof. Three sets of specific primers and Taqman probes as well as positive controls specific to African swine fever virus CP530R genes, swine fever virus 5 minute-UTR genes and swine respiratory syndrome virus NSP2 genes respectively are designed and synthesized, and a rapid, easy and convenient triple fluorescent RT-PCR detection system with high specificity and high sensitivity is established by using the three sets of primers and probes, so that nucleic acids of the African swine fever viruses, the swine fever viruses and the respiratory syndrome viruses can be detected synchronously from a detected sample within 3-4 hours in a rapid, accurate, specific, safe, easy and convenient way. The detection reagent can be applied to synchronous detection of the nucleic acids of trace African swine fever viruses, swine fever viruses and respiratory syndrome viruses in hogs and relevant samples thereof.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Human virus causing severe acute respiratory syndrome (SARS) and uses thereof
ActiveUS20100080824A1Animal cellsSsRNA viruses positive-senseNucleotideSevere acute respiratory syndrome
The present invention relates to an isolated novel virus causing Severe Acute Respiratory Syndrome (SARS) in humans (“hSARS virus”). The hSARS virus is identified to be morphologically and phylogenetically similar to known member of Coronaviridae. The present invention provides the complete genomic sequence of the hSARS virus. Furthermore, the invention provides the nucleic acids and peptides encoded by and / or derived from the hSARS virus and their use in diagnostic methods and therapeutic methods, including vaccines. In addition, the invention provides chimeric or recombinant viruses encoded by said nucleotide sequences and antibodies immunospecific to the polypeptides encoded by the nucleotide sequences.
Owner:VERSITECH LTD
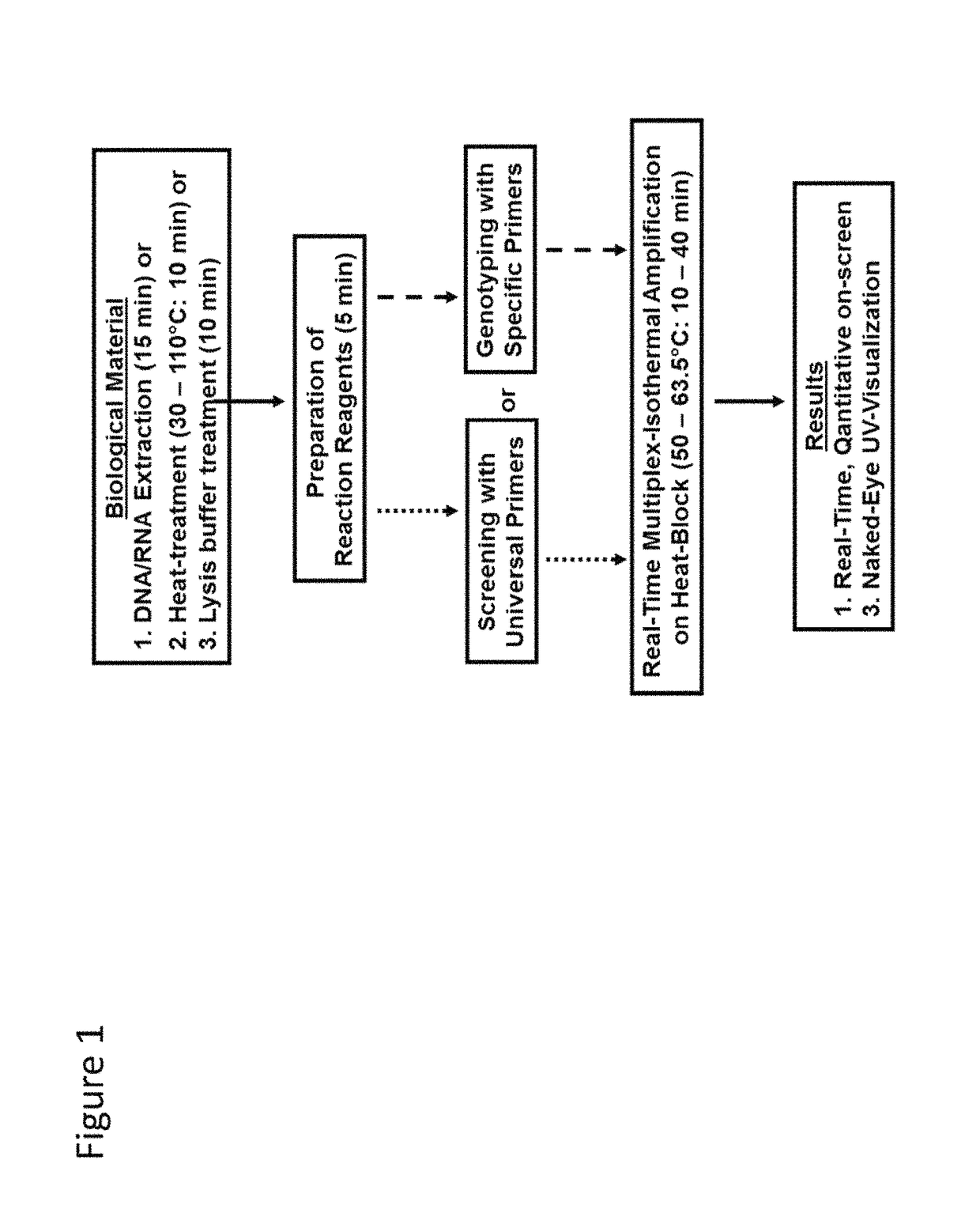
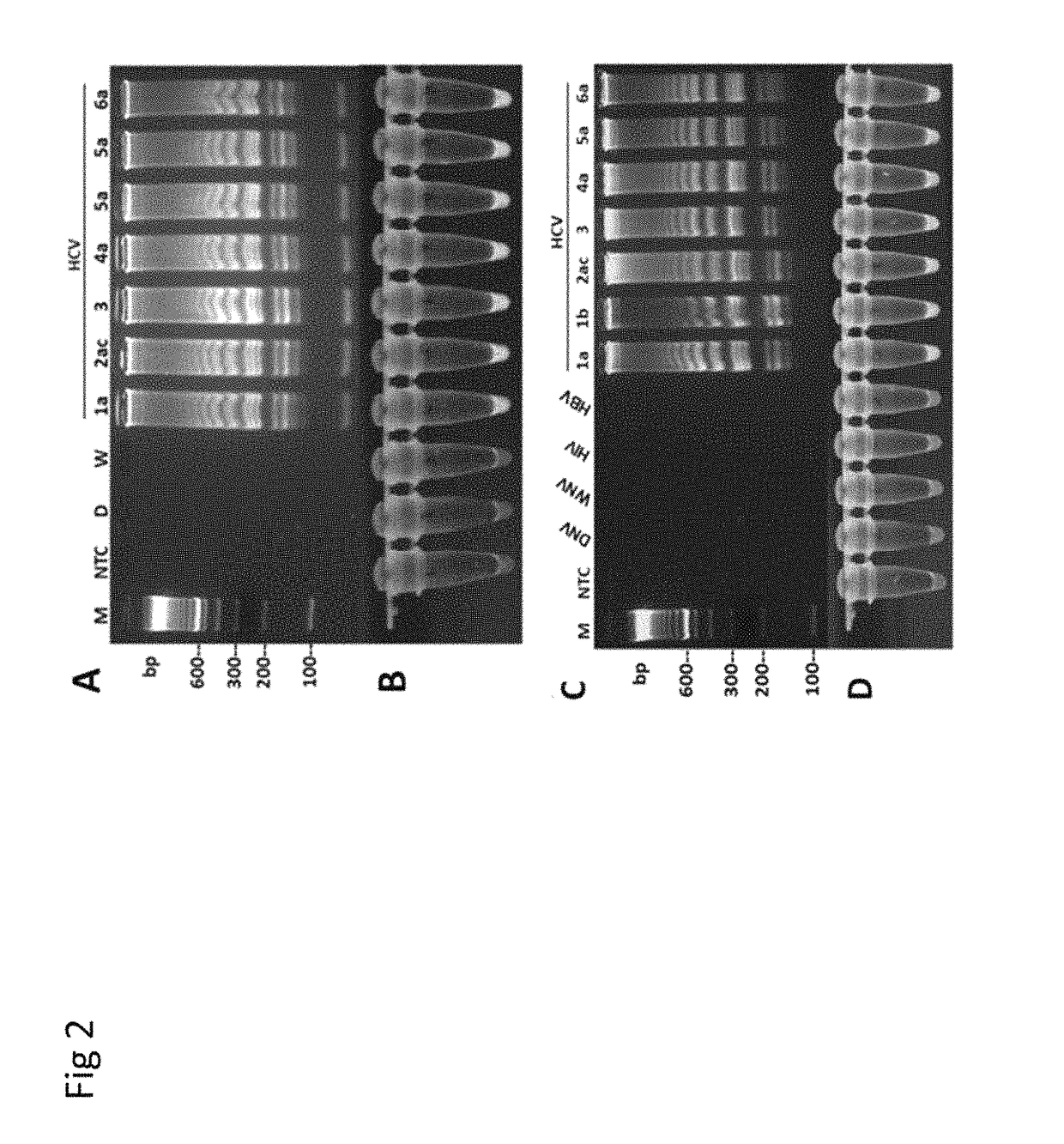
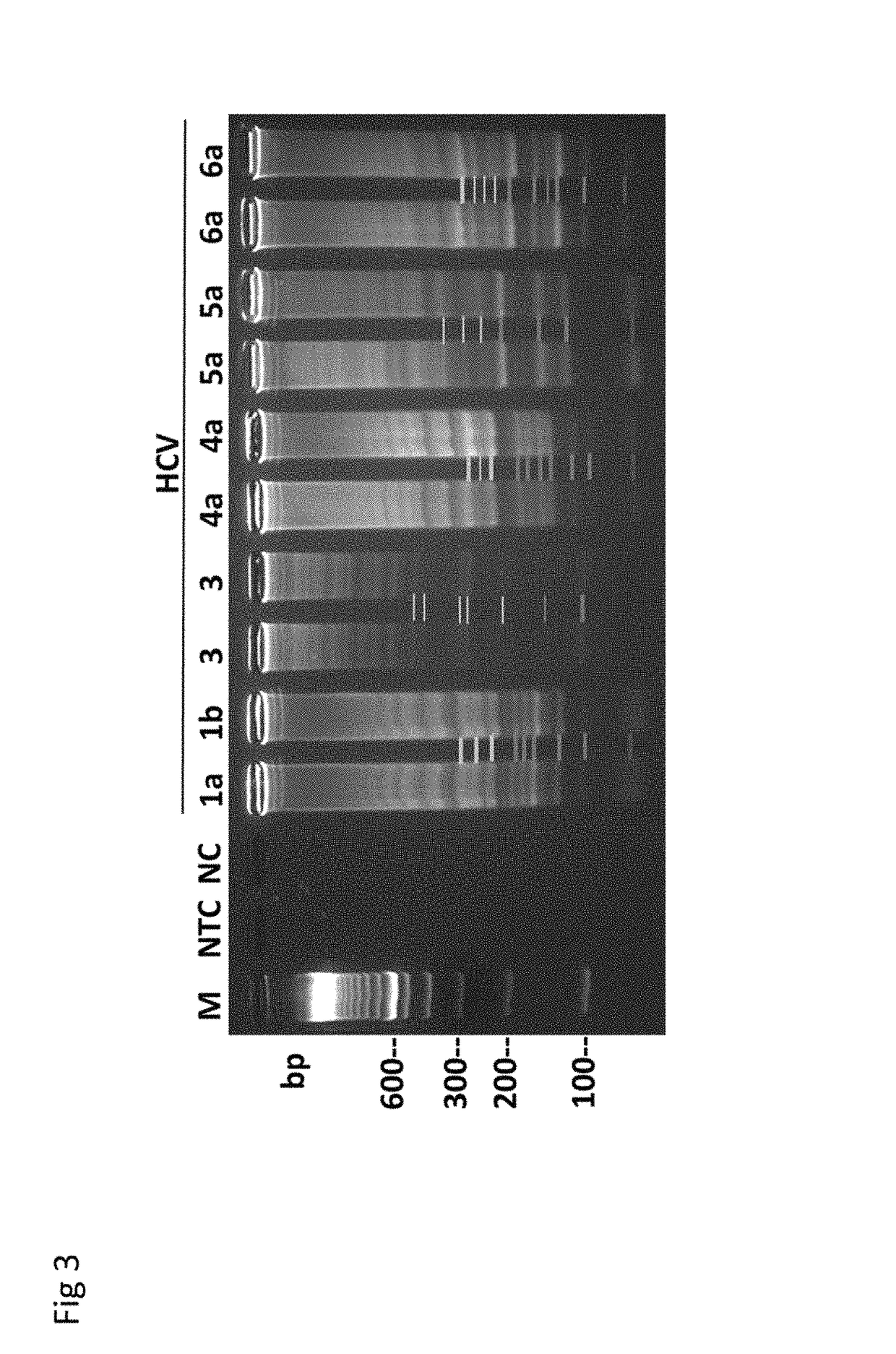
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
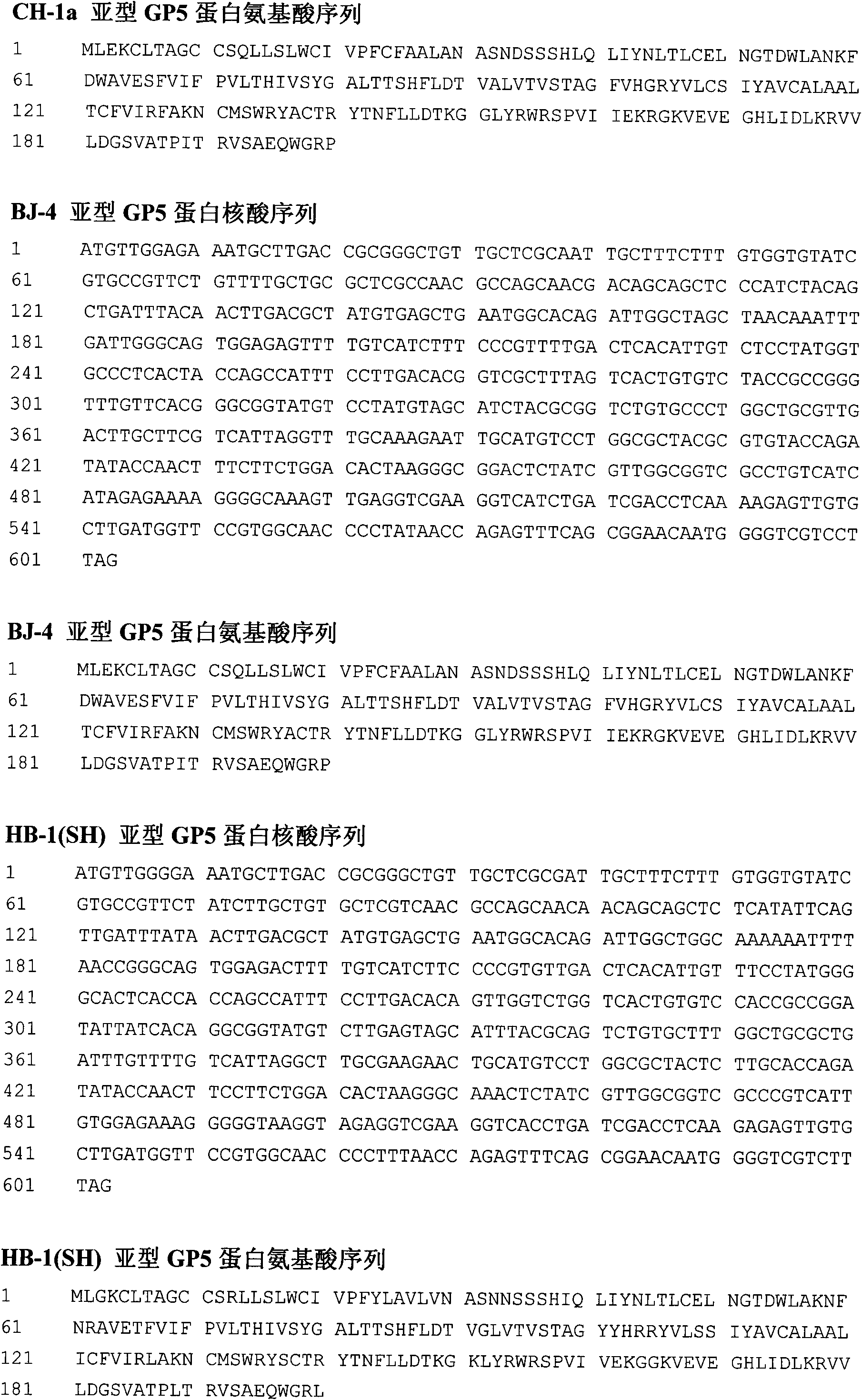
Porcine reproductive and respiratory syndrome virus-like particle vaccine and preparation method thereof
A porcine reproductive and respiratory syndrome virus-like particle vaccine and a preparation method thereof relate to the field of biological medicines and aims at disclosing the porcine reproductive and respiratory syndrome virus-like particle vaccine (PRRS VLP vaccine) and the preparation method the vaccine. The PRRS VLP vaccine contains a VLP comprising three structure proteins of porcine reproductive and respiratory viruses M, N and GP5 and can excite favorable dual cell and humoral immune response. By adding or not adding an adjuvant into the formed VLP protein antigen, the pharmacodynamic test shows the prepared injection type, nasal drop type and water agent type vaccines are immunized with different animal groups so as to safely and effectively prevent the PRRSV infection and provide ideal vaccines for safely and effectively immunizing, preventing and controlling the PRRSV infection on different groups of sows, piglets and fat pigs.
Owner:CHONGQING UNIV
Synthetic peptide vaccine for treating porcine reproductive and respiratory syndrome and preparation method thereof
InactiveCN101845083AImprove securityImprove efficiencyViral antigen ingredientsVirus peptidesMedicinePorcine reproductive and respiratory syndrome virus
The invention provides a synthetic peptide vaccine for treating porcine reproductive and respiratory syndrome and a preparation method thereof, in particular relates to polypeptide of the synthetic peptide vaccine for treating the porcine reproductive and respiratory syndrome and a vaccine which contains the polypeptide and a method for preparing the polypeptide and the vaccine. The amino acid sequence of the polypeptide is an amino acid sequence shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 or SEQ ID No.4. The vaccine prepared from the polypeptide can effectively cope with the antigenic variation of a porcine reproductive and respiratory syndrome virus, ensures biological safety, is easy for large-scale synthesis and has good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Reagents and Methods for Detecting Severe Acute Respiratory Syndrome Coronavirus
InactiveUS20080044814A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSevere acute respiratory syndrome coronavirusPediatrics
This invention provides reagents and methods for detecting the severe acute respiratory syndrome coronavirus (SARS CoV). This invention also provides related compositions, kits, systems, and computers.
Owner:GENOME INST OF SINGAPORE
Pig propagation and breathing syndrome virus stain, and inactivated vaccine prepared thereby
ActiveCN101376878AGood emulsifying effectLow costViral antigen ingredientsInactivation/attenuationMicroorganismPorcine reproductive and respiratory syndrome virus
The invention discloses a separated porcine reproductive and respiratory syndrome virus JJ strain with the microorganism collection number of CGMCC NO.2129, an inactivated vaccine prepared by the virus strain and a preparation method thereof. The porcine reproductive and respiratory syndrome virus inactivated vaccine has good immune protection effectiveness, and can effectively prevent porcine reproduction and respiratory syndrome.
Owner:华威特(江苏)生物制药有限公司
Method of preparing pig replication and respiration syndrome deactivation concentrated vaccine 'SD1 strain'
InactiveCN101234198ANo side effectsImprove immunityViral antigen ingredientsInactivation/attenuationALUMINUM STEARATESPorcine reproductive and respiratory syndrome virus
The invention provides a preparation method of an inactivated and concentrated vaccine 'SD1 strain' for a Porcine reproductive and respiratory syndrome, which is prepared with a water phase and an oil phased according to the following weight percentage content: the water phase is prepared by fully mixing 96 shares of 'SD1 strain' virus culture solution, which is American Porcine reproductive and respiratory syndrome that is inactivated for 20 hours and concentrated 2 times, with 4 shares of Tween minus 80, which occupies 33 percent of the vaccine; the oil phase is prepared by mixing 94 shares of No. 10 white oil with 6 shares of Span minus 80 and then adding a 2 percent aluminum stearate according to the total amount to stir to be transparent, and sterilizing with a high pressure at a temperature of 116 DEG C, which occupies 67 percent of the total amount of the vaccine. The vaccine, with a preservation period of 12 months, is safe and reliable to the Porcine reproductive and respiratory syndrome easily infected animals, and is suitable for pigs of different species and various day old, the immunity protection rate of which reaches 80 percent above, the immunity period of validity of which continues more than 6 months. The safety and immunity efficacy of the vaccine reaches an advanced level among the similar products in the world.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Pig replication and respiration syndrome virus attenuated vaccine strain and uses thereof
ActiveCN101220348AResist attackImprove securityViral antigen ingredientsAntiviralsMicroorganismPorcine reproductive and respiratory syndrome virus
The invention discloses a porcine reproductive and respiratory syndrome virus attenuated vaccine strain, and the microorganism collection number is CGMCC NO.1883. The attenuated vaccine strain of the invention has stable genetic variation and good safety, which is safe and non-pathogenic for pregnant sows, piglets and the pigs at various ages. The immunized piglets after using the attenuated vaccine strain of the invention for immunization can resist the attack of the strong virus of the porcine reproductive and respiratory syndrome virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Compounds and methods for treating respiratory diseases
InactiveUS20110269834A1Reduce developmentLittle and no accompanying cytotoxicityBiocideOrganic chemistryBovine respiratory diseaseRespiratory disease
Described herein are compounds and compositions, and methods for using the compounds and compositions, for treating respiratory diseases and illness, such as severe acute respiratory syndrome (SARS).
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Viral inactivation process
ActiveUS20110020406A1Reduce viremiaSsRNA viruses positive-senseViral antigen ingredientsFamily ArteriviridaePorcine reproductive and respiratory syndrome virus
The invention relates generally to the field of virology. More particularly, the present invention relates to methods for determining the effect of a viral inactivation procedure on the antigenicity of the inactivated virus. In particular for a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, in particular for Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). The invention further provides methods to determine the antigenicity of an inactivated virus as well as methods to screen for anti-viral compounds using any one of the aforementioned methods Methods of using the inactivated and immunogenic virus thus obtained, in particular in the manufacture of a vaccine are also provided by the present invention.
Owner:UNIV GENT
RT-PCR (Reverse Transcription-Polymerase Chain Reaction) method for detecting porcine reproductive and respiratory syndrome virus (PRRSV) classical strains, high-pathogenicity variant strains and NADC-30 strains
InactiveCN106868224ADirect and efficient diagnosisDirect and effective controlMicrobiological testing/measurementDNA/RNA fragmentationHighly pathogenicPorcine reproductive and respiratory syndrome virus
The invention discloses an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) method for detecting porcine reproductive and respiratory syndrome virus (PRRSV) classical strains, high-pathogenicity variant strains and NADC-30 strains. A pair of primers are designed by applying Primer5.0 software through referring to a PRRSV Nsp2 gene sequence published in GenBank. The pair of primers can be used for detecting three subtypes with different amino acid quantities of a PRRSV through an RT-PCR method. The RT-PCR method disclosed by the invention has high sensitivity and good specificity, and can be used for successfully detecting the three subtypes of the PRRSV, such as the classical strains, the variant strains ad the NADC-30 strains, and can be used for detecting clinical samples with the suspected PRRSV, so that the RT-PCR method is used for diagnosing the PRRSV and carrying out epidemiological monitoring.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Reagent kit for detecting porcine reproductive and respiratory syndrome virus and detecting method
ActiveCN101463395AGood removal effectQuick checkMicrobiological testing/measurementDNA/RNA fragmentationRespiratory syndrome virusEnzyme inhibitor
The invention relates to a reagent kit used for detecting porcine reproductive and respiratory syndrome virus and a detection method thereof. The reagent kit comprises a RNA enzyme inhibitor, AMV revertase, Taq enzyme, RNA-free enzyme solution, One Step RNA PCR mixed solution and a mixture of primers with negative control and sequence selected from SEQ ID NO: 1 to SEQ ID NO:6. The detection method comprises the step of using the reagent kit to carry out RT-PCR amplification. The method can detect the porcine reproductive and respiratory syndrome virus fast, sensitively and exactly with low cost.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com