Pig propagation and breathing syndrome virus stain, and inactivated vaccine prepared thereby
A respiratory syndrome and inactivated vaccine technology, applied in the field of virus strains, porcine reproductive and respiratory syndrome virus strains, inactivated vaccines and their preparation, can solve problems such as inability to provide effective protection and achieve good emulsification effect , Small side effects, easy injection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Isolation and acquisition of PRRSV TJ strain
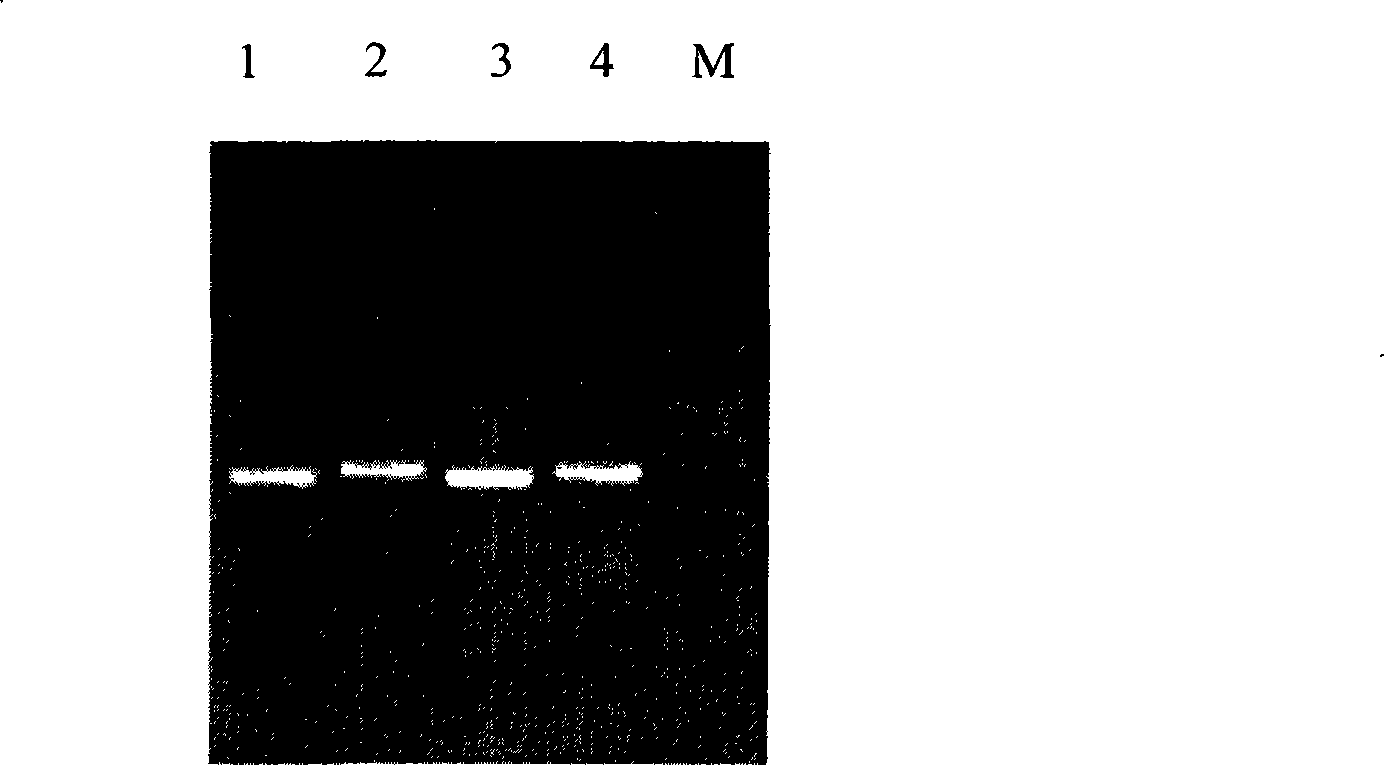
[0023] A strain of virus was isolated from the diseased material collected from a dead pig in a pig farm with high fever in Tianjin. After blind passage of Marc-145 cells for 2 generations, it showed characteristic PRRS cell lesions. After RT-PCR detection, both samples amplified The target fragments of 433bp and 468bp were found, which proved that the isolate was American type (see figure 1 ). Returning the isolated virus cell culture to animals produces PRRS-specific clinical symptoms, high fever, skin flushing, loss of appetite, depression, hind limb lameness or even paralysis, mild dyspnea, and the incidence rate is 100%. The virus was isolated again from the test animal, and its GP5 and Nsp2 genes were amplified and sequenced. The results showed that the strain was a mutated PRRSV, and the strain was named PRRSV TJ strain. The sequence is shown in SEQ ID NO: 1, and the strain is submitted for deposit, and i...
Embodiment 2
[0024] Example 2 Purification and isolation of virus
[0025] The PRRSV TJ strain was diluted 10 times serially, and the dilution range was 10 -2 -10 -6 , after dilution, inoculate a 6-well cell culture plate covered with a single layer of Marc-145 cells, 1ml / well, absorb for 1h, discard the virus solution, wash twice with serum-free MEM, add 2× nutrient solution and sterilized agar solution Mixed solution, 3ml / well, after cooling at room temperature, place at 37°C 5% CO 2 Cultivate in an incubator for 3-4 days, observe the plaques, and pick a single plaque in a well with fewer plaques for passage.
Embodiment 3
[0026] Example 3 PRRSV inactivation and inactivation effect test
[0027] (1), PRRSV inactivation
[0028] (1) Cell culture: culture Marc-145 cells in spinner bottles to make the cell density reach 5-10×10 7 / ml;
[0029](2) virus inoculation: be 0.01-0.5 PRRSV TJ strain of the present invention is inoculated in the Marc-145 cell cultured of step (1) with infectious dose (MOI), cultivate 3-5 days, when cytopathic changes reaches 80% Harvest the virus fluid;
[0030] (3) Add BEI to the virus liquid harvested in step (2), the final concentrations are 1mM, 2mM and 3mM respectively, make it fully mixed, and then place it at 37°C for inactivation, before adding BEI (0h) and after adding BEI Samples were collected at 30min, 1h, 2h, 4h, 8h, 12h, 24h, and 28h (10ml / time), and the collected samples were used for virus titer determination, and the inactivation kinetic curve was drawn. Determine the final concentration of BEI and the optimal inactivation time, see the kinetic curve o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com