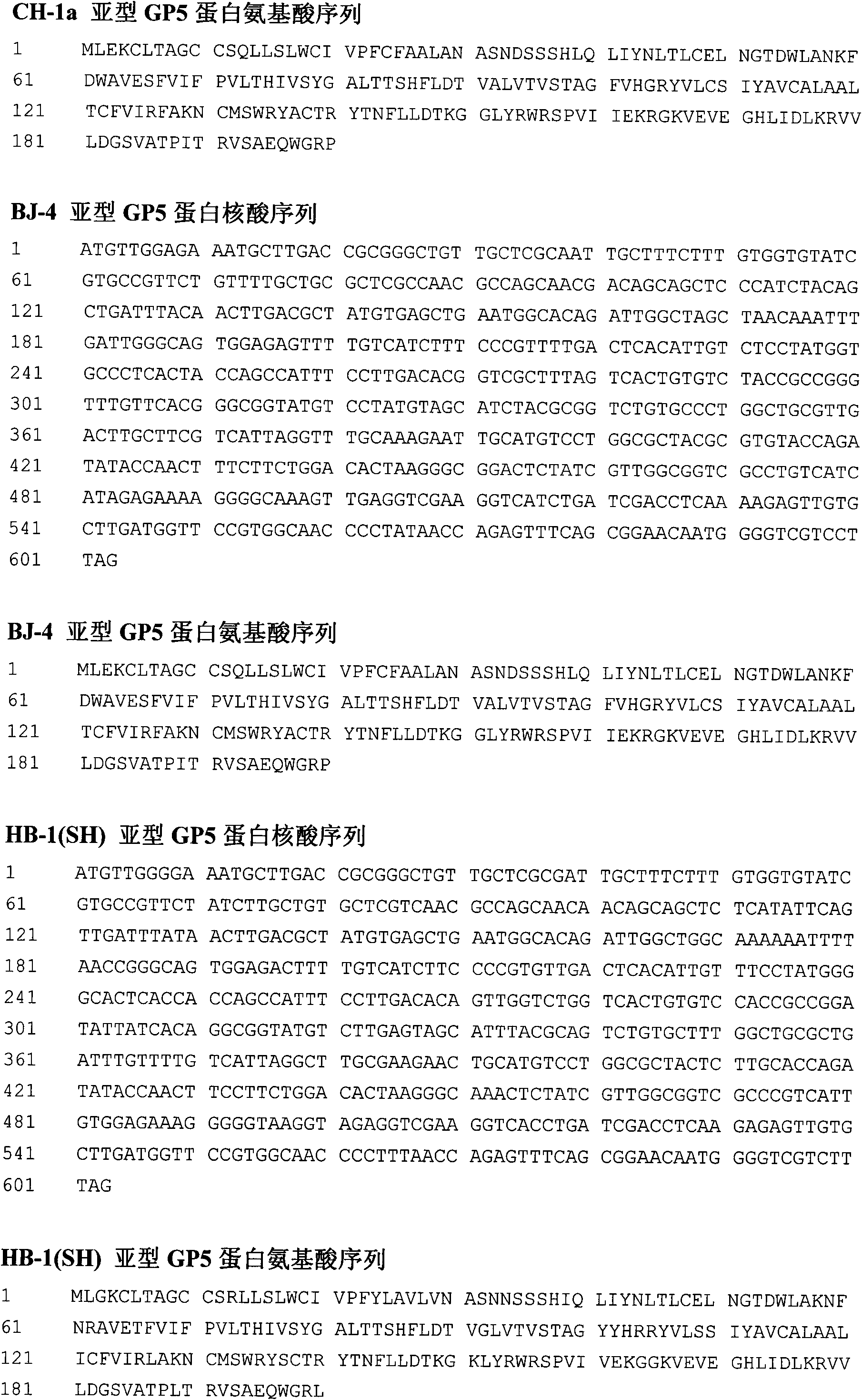
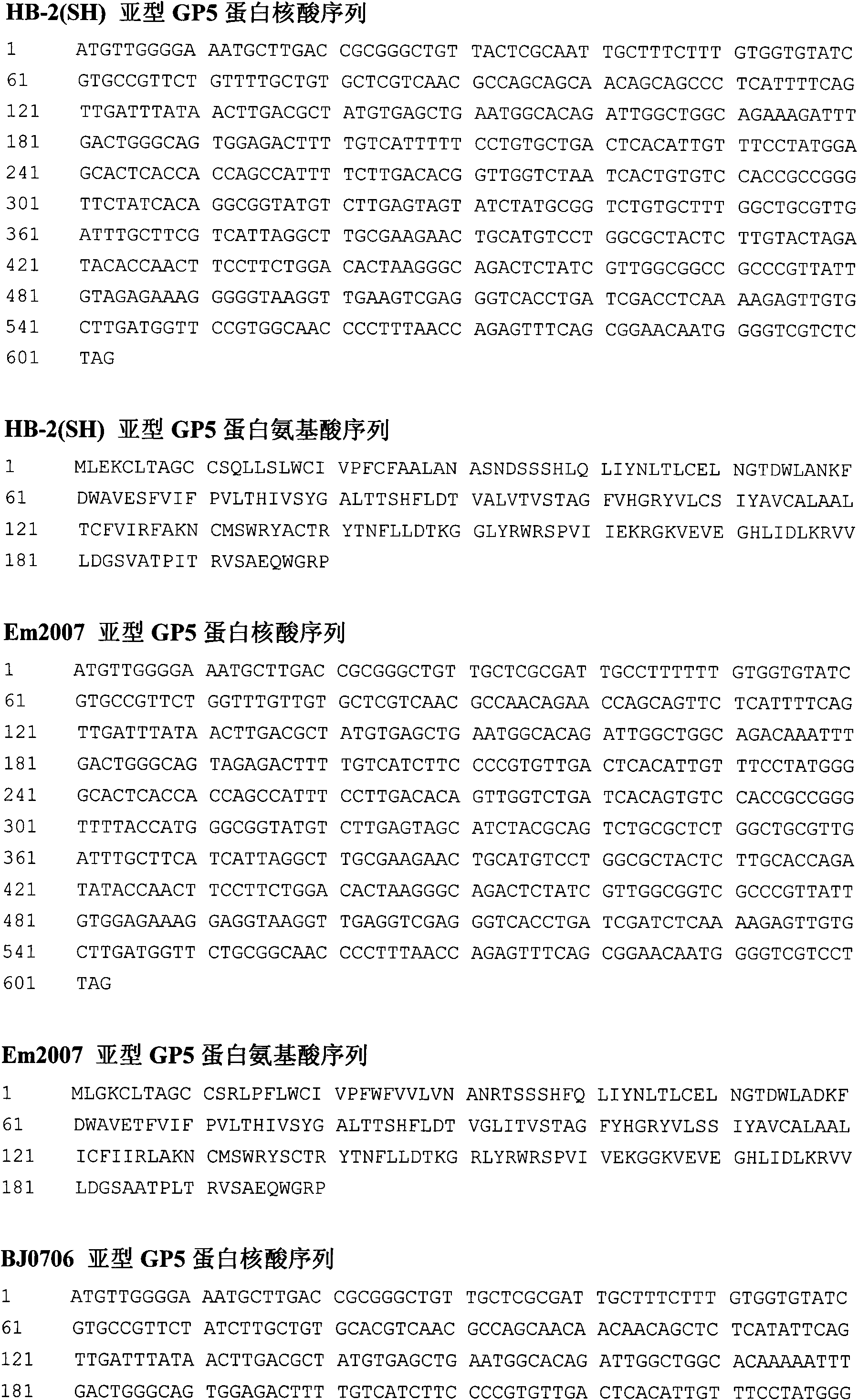
Porcine reproductive and respiratory syndrome virus-like particle vaccine and preparation method thereof
A technology for respiratory syndrome and pig reproduction, applied in biochemical equipment and methods, antiviral agents, viruses/phages, etc., can solve the problems of no potential danger, long preparation period and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0030] Experimental example 1: PRRS VLP vaccine verification experiment of the present invention
[0031] The PRRS VLP vaccine injection, nasal drops, and drinking water formulations prepared in Example 8 of the present invention are uniform in appearance, without peculiar smell and bacterial contamination; they have no pyrogen, abnormal toxicity and acute toxicity through guinea pig and rabbit tests; Intracerebral inoculation in rats and intramuscular injection in the neck of 3-day-old piglets showed no signs of disease; the protein content determined by the Lorry method was within the dose range; the diameter of VLP was observed by electron microscopy to be about 45-65nm, which was consistent with natural viruses; the DNA content was determined by ELISA All were below 50EU; the presence of antigenic protein components was detected by SDS-PAGE and WB; the neutralizing antibody titer in the serum of Balb / C mice once immunized was above 3200.
experiment example 2
[0032] Experimental Example 2: Effect experiment of the PRRS VLP vaccine of the present invention on the immune response in experimental pigs
[0033]The PRRS VLP vaccine prepared in Example 8 of the present invention was added or not added to the corresponding adjuvant prepared injection, nasal drip, and drinking water vaccine dosage forms, and PBS, aluminum salt, nano-aluminum, P3BSK4, rLT, were used as the control group, and were immunized according to their respective routes. 3 days old piglets, and its immunization dosage of back-up sows are immunized twice (0, 21 days) by embodiment 8 vaccine low dosage group; Collect the peripheral blood of each group piglets and sows 14 days after the last immunization, separate For serum and immune cells, spleen lymphocytes, lung and nasal lavage fluid were collected; the serum antibody titer was determined to be over 6400 by ELISA method, and the neutralizing antibody titer was determined to be over 3200 by MTT method, and was measure...
experiment example 3
[0034] Experimental Example 3: Experiment of the immune protection effect of the PRRS VLP vaccine of the present invention on experimental pigs
[0035] The PRRS VLP vaccine prepared in Example 8 of the present invention was prepared with or without adding corresponding adjuvants in the form of injection, nasal drops, and drinking water as the experimental group, and the virus was inactivated with PBS, aluminum salt, nano-aluminum, P3BSK4, rLT, and PRRSVCH-1a Be a control group, immunize piglets and gilts at the age of 3 days by their respective approaches, and their immunization doses are immunized twice (0, 21 days) by the vaccine low-dose group in Example 8, and each test group, The experimental pigs in the control group used 10 5.0 TCID 50 Clinically isolated PRRSV strains were challenged by intramuscular injection in the neck to observe the protective effect. The result shows that PRRS VLP vaccine of the present invention all can produce high-level protection effect, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com