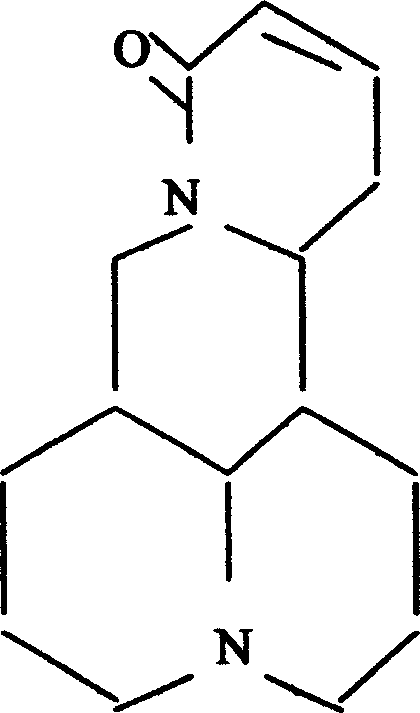
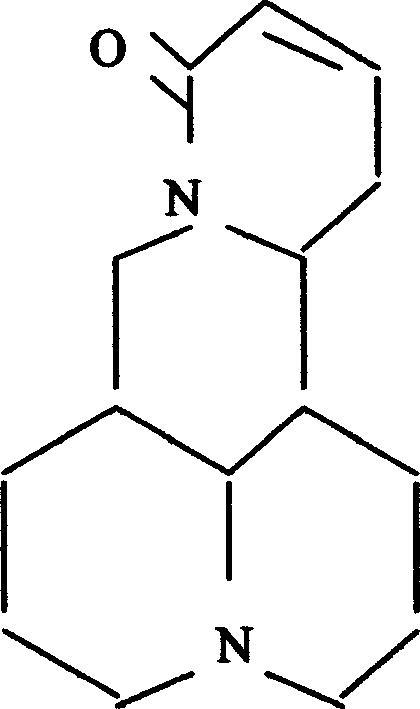
Application of l-sophocarpine on preparing medication for curing disease caused by coronavirus
A coronavirus, sophocarpine technology, applied in the directions of drug delivery, antiviral agents, drug combination, etc., to achieve the effects of simple operation, less toxic and side effects, and abundant drug sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Extraction of Sophocarpine and Preparation of Injection
[0030] Grind 10kg of Sophora Sophora (including leaves, flowers, and stems), add 5% ammonium hydroxide to moisten, heat and extract under reflux in chloroform, and extract with 8% sulfuric acid to obtain the total alkaloids; extract the total alkaloids with toluene, and pass through sufficient Extract sophocarpine with 7% hydrochloric acid, adjust the pH to 8, filter with suction, and then recrystallize with acetone to obtain the fine product of sophocarpine with a yield of 0.12%.
[0031] Dissolve a certain amount of sophocarpine in water for injection, filter and measure the content, adjust the pH to 4.0-4.5, then fill and seal through a 0.65um microporous membrane, and finally sterilize with flowing steam at 100°C for 30 minutes. Injectable formulations are available.
Embodiment 2
[0032] Example 2 Sophocarpine drug anti-SARS virus activity screening (virus-cell level model)
[0033] The test sample is sophocarpine; the virus strain is BJ-01, which comes from the Institute of Microbiology and Epidemiology, Beijing Academy of Military Sciences.
[0034] 1. Preliminary experiment
[0035] Vero-E6 cells were used as virus host cells (susceptible cells), and the effect of samples against virus-infected cells was detected, and the detection indicators were cell pathogenic effect (Cell Pathogenic Effect, CPE) and the protection rate of infected cells.
[0036] The sophocarpine sample was dissolved in culture medium or DMSO to prepare an appropriate initial concentration, 2-fold dilution, and 7 dilutions.
[0037] Seed Vero-E6 cells in a 96-well culture plate at 37°C, 5% CO 2 After culturing, after the SARS virus infects the cells, samples of different dilution concentrations are added respectively, and a virus control, a cell control and a sample control are s...
Embodiment 3
[0054] Example 3 Sophocarpine pharmacological experiment on animals
[0055] 1. Dog
[0056] In canine animals, a floating catheter was inserted into the pulmonary artery through the head and neck or femoral veins, and then into the pulmonary capillaries to continuously monitor the pulmonary capillary wedge pressure and dynamically observe the effect of sophocarpine on the change of the pulmonary capillary wedge pressure. It can reduce the already raised capillary wedge pressure to normal levels.
[0057] After dog A was anesthetized and intubated, the blood pressure was 149 / 89mmHg, HR(EKG) was 148 / min, PCWP was 17-18mmHg and remained stable. Add 6mg / kg sophocarpine injection into 5% GS20ml, inject slowly at a rate of 1ml per minute, PCWP drops from 17mmHg to 9mmHg, and rises to 13mmHg after 10 minutes, and then injects 8mg / kg sophocarpine after 20 minutes The injection was added with 5% GS20ml and injected slowly at a rate of 1ml per minute. PCWP did not continue to decline...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com