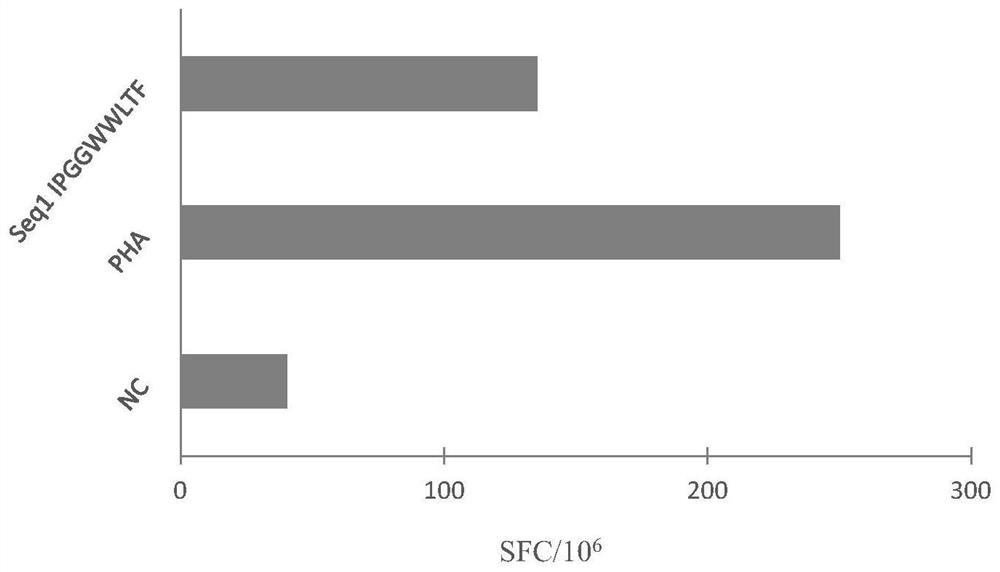
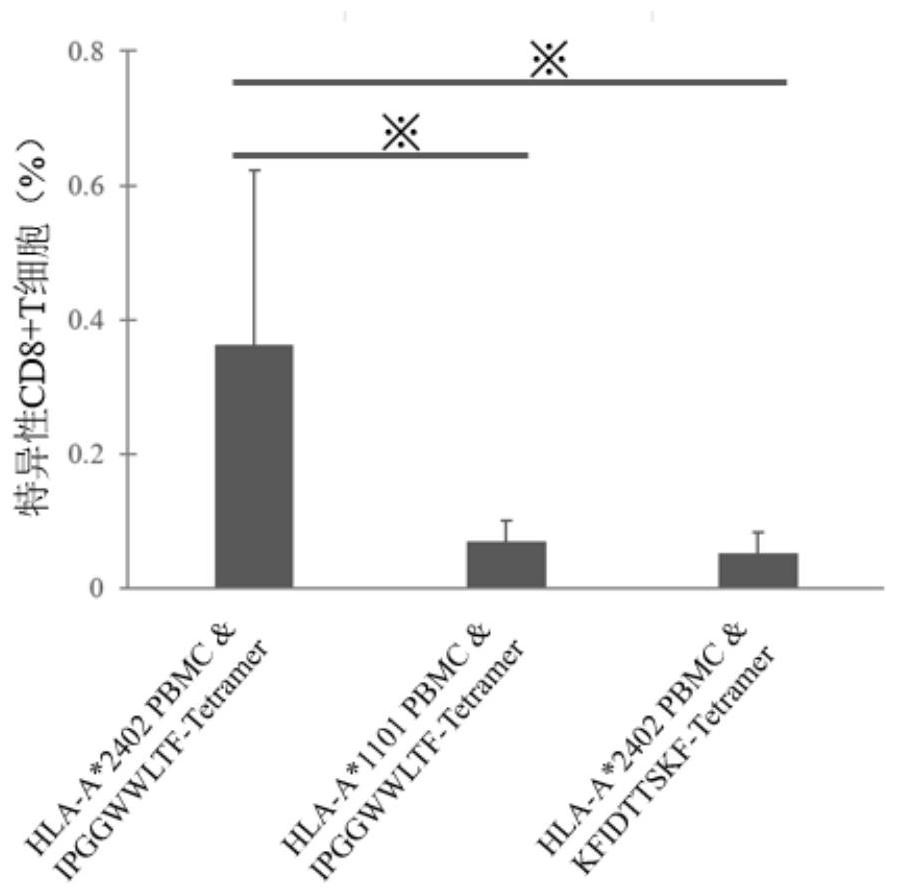
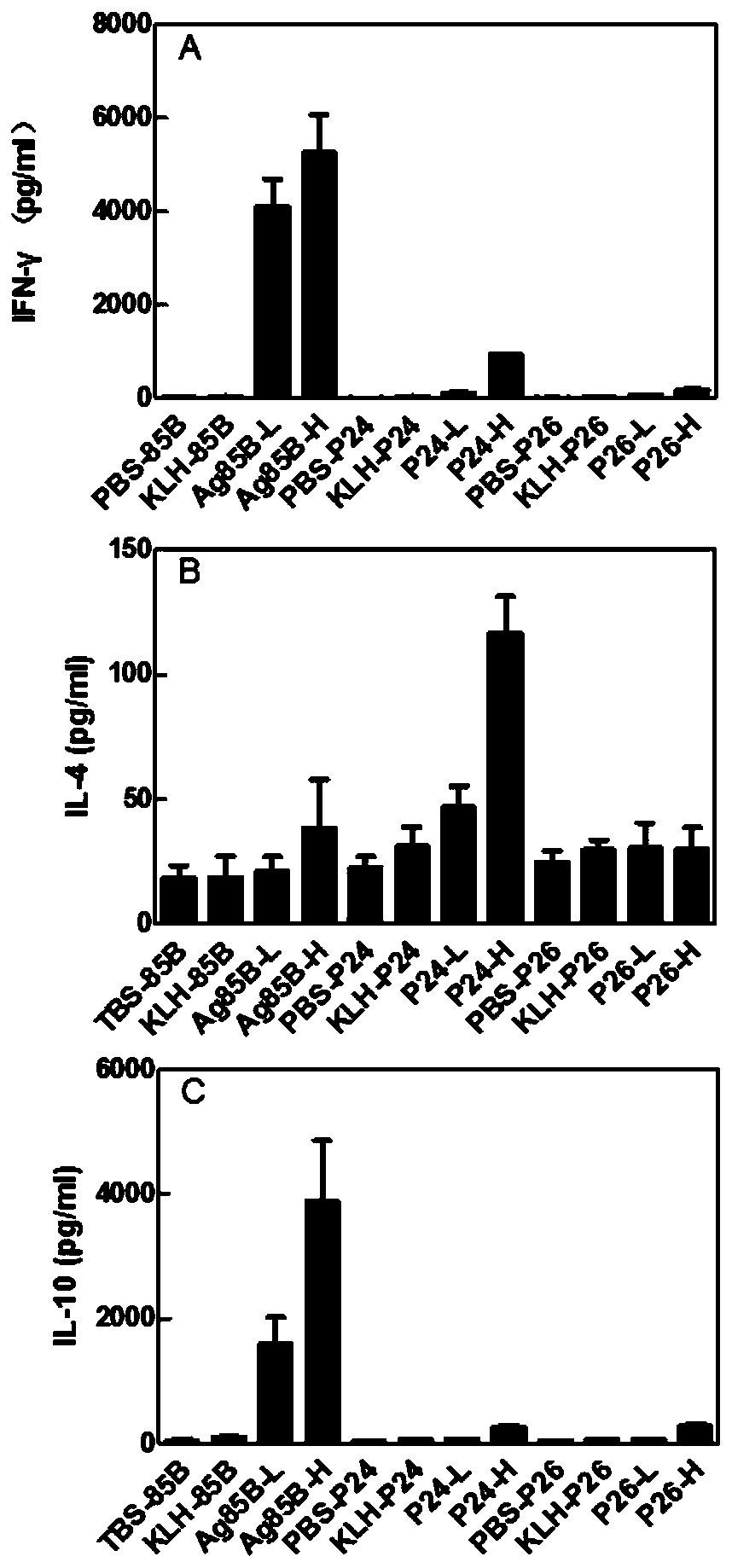
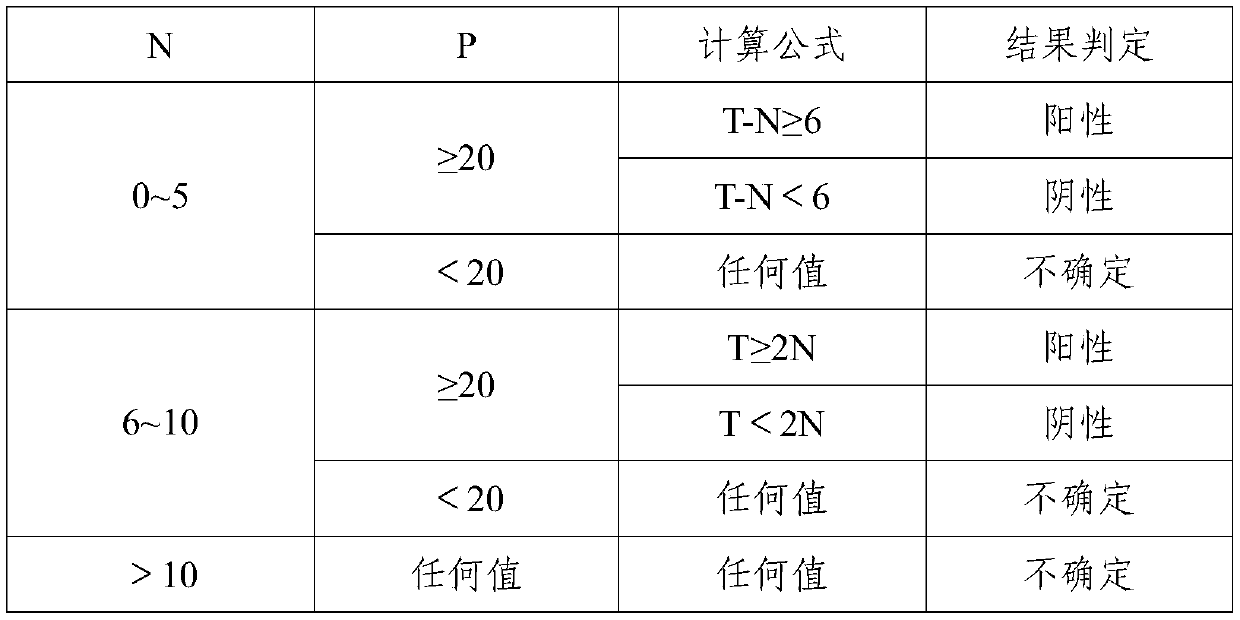
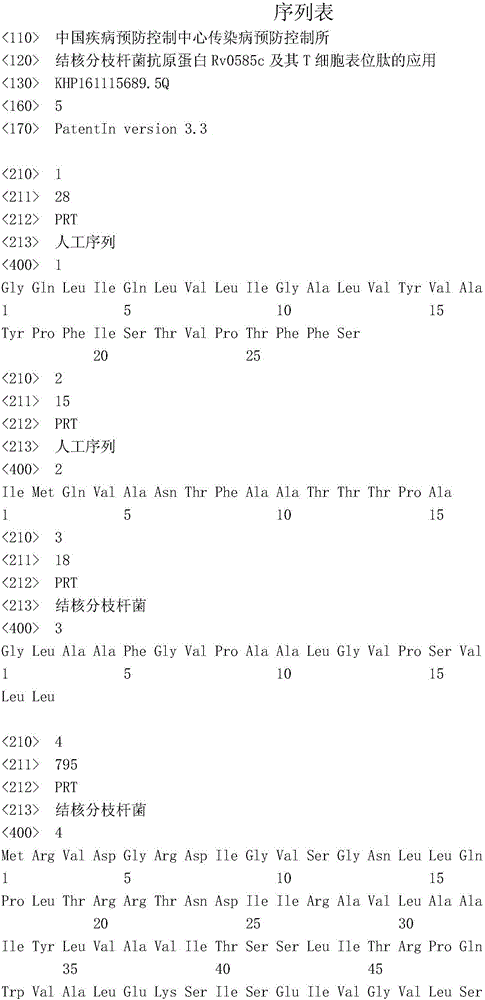Patents
Literature
34 results about "Epidemiological Monitoring" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Collection, analysis, and interpretation of data about the frequency, distribution, and consequences of disease or health conditions, for use in the planning, implementing, and evaluating public health programs.
Fluorescent PCR (polymerase chain reaction) detection kit for babesia caballi disease
InactiveCN105734158AEradicate hazardsPromote healthy developmentMicrobiological testing/measurementDNA/RNA fragmentationPositive controlEpidemiological Monitoring
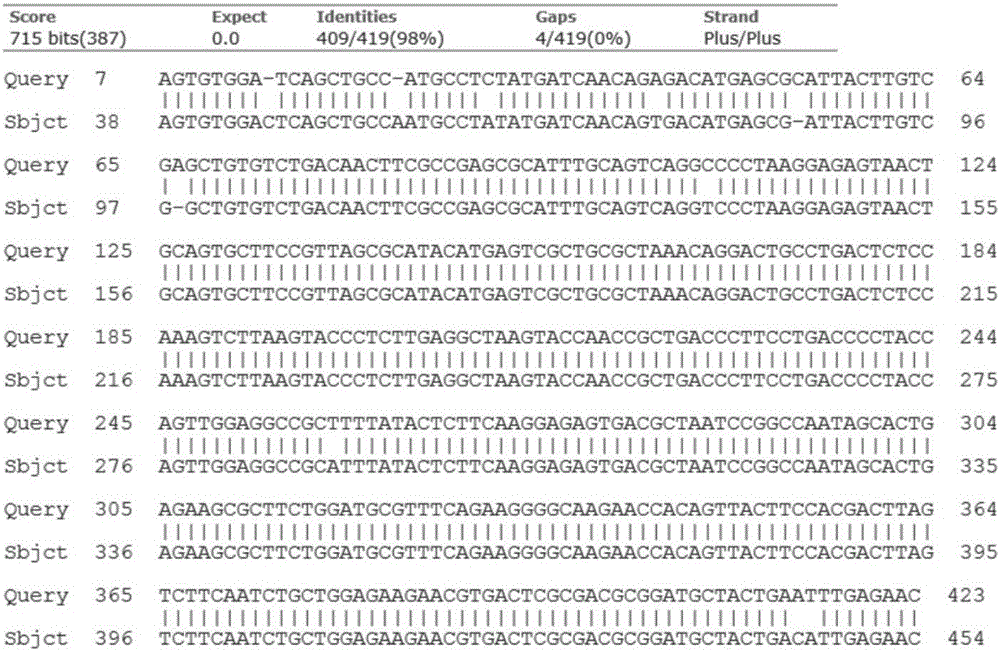
The invention belongs to the technical field of biological detection, and in particular relates to a babesia caballi disease detection kit based on a fluorescent quantitative PCR (polymerase chain reaction) scalar quantity positive control, which is used for clinical diagnosis and daily monitoring of equine animal infected babesia caballi disease. By designing detection primers as shown by SEQ ID No.1 and SEQ ID No.2 and a fluorescent probe as shown by SEQ ID No.3, combining absolute quantification analysis of a fluorescent quantitative PCR system, and optimizing a reaction system and cycling conditions, a fluorescent PCR detection method for the babesia caballi disease is established. Moreover, the fluorescent PCR detection kit for the babesia caballi disease based on a fluorescent quantitative PCR scalar quantity is established, and is suitable for clinical diagnosis and epidemiological monitoring of the babesia caballi disease.
Owner:XINJIANG AGRI UNIV
On-site rapid high-sensitivity detection kit of prawn infectivity muscle necrosis virus and detection method thereof
InactiveCN101921873AShorten the timeShorten detection timeMicrobiological testing/measurementDiseaseTE buffer
The invention relates to an on-site rapid high-sensitivity detection kit of prawn infectivity muscle necrosis virus and a detection method thereof. The detection kit comprises a sampling tube, a rinsing tube filled with distilled water, a nucleate degeneration tube filled with a TE buffer solution, an amplification detection tube filled with amplification reaction liquid and nucleic acid dye, a negative control tube, a positive control tube, an FTA membrane, rapid drying liquid, and the like. Compared with the RT-PCR detection method, the detection method in the invention has the advantages of higher specificity, sensitivity and convenience, low cost, convenient use, more accurate and rapid detection and extremely sensitivity, is safe to both human and the environment and can substitute the traditional relevant detection method. The invention can be used in both the indoor laboratory and outdoor production field, has great significance for strengthening the epidemiological monitoring of prawn infectivity muscle necrosis virus, prawn cultivation waterbody monitoring and disease prevention and control and has great promotion and application value.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
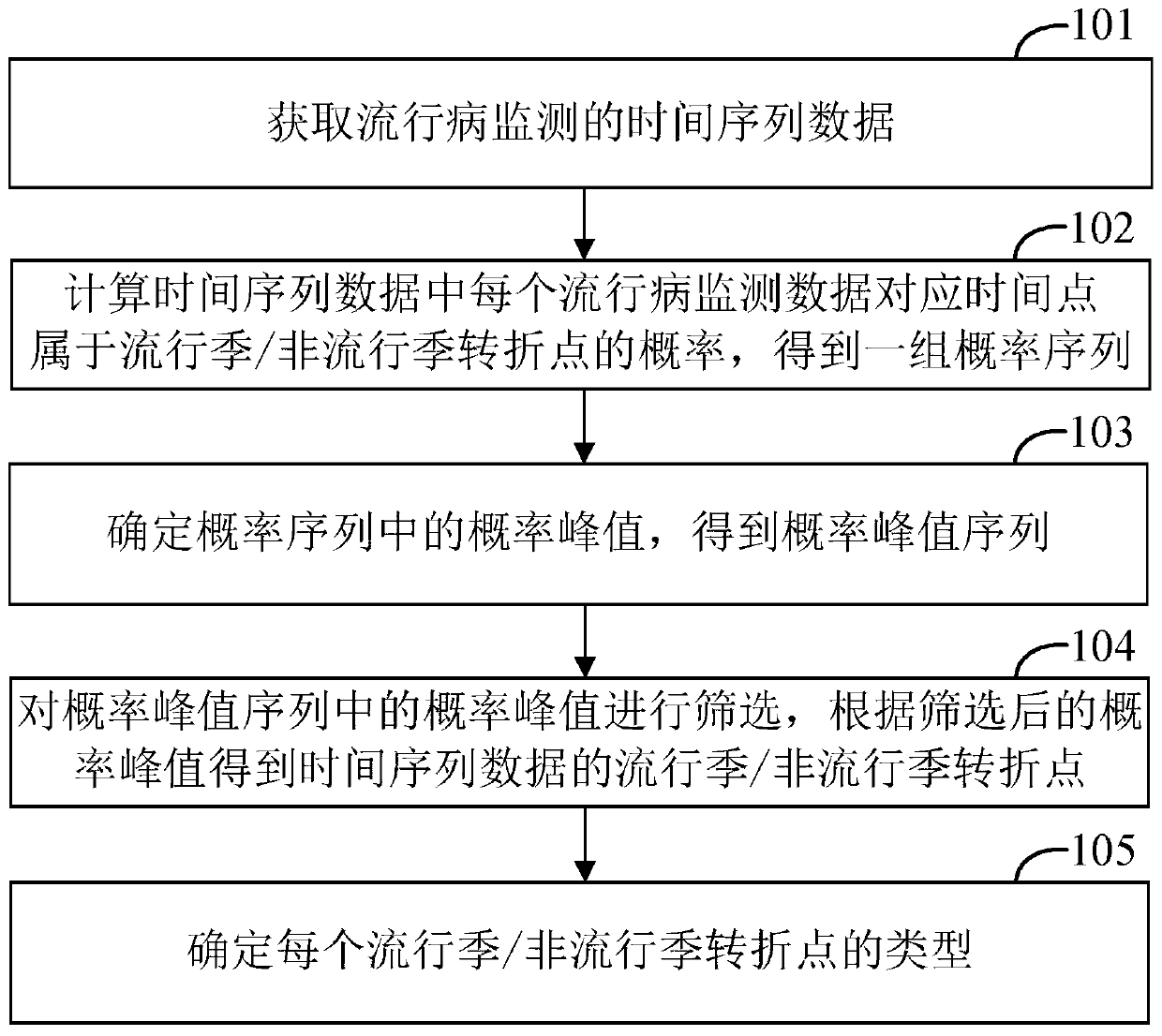
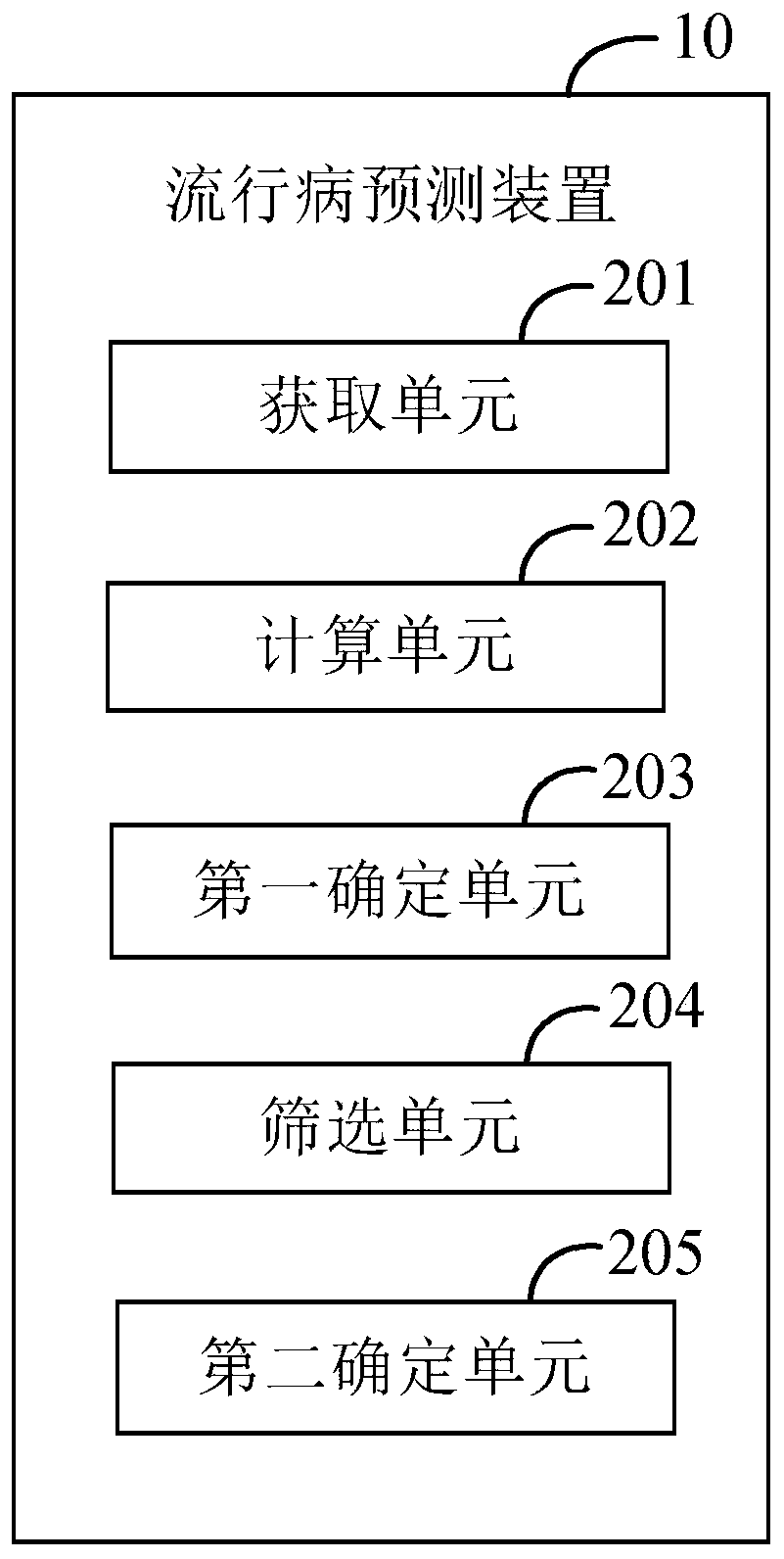
Epidemiological prediction method, computer device, and computer readable storage medium
ActiveCN108630321AEfficient forecastingAvoid spending a lot of timeMedical data miningEpidemiological alert systemsEpidemiological MonitoringAlgorithm
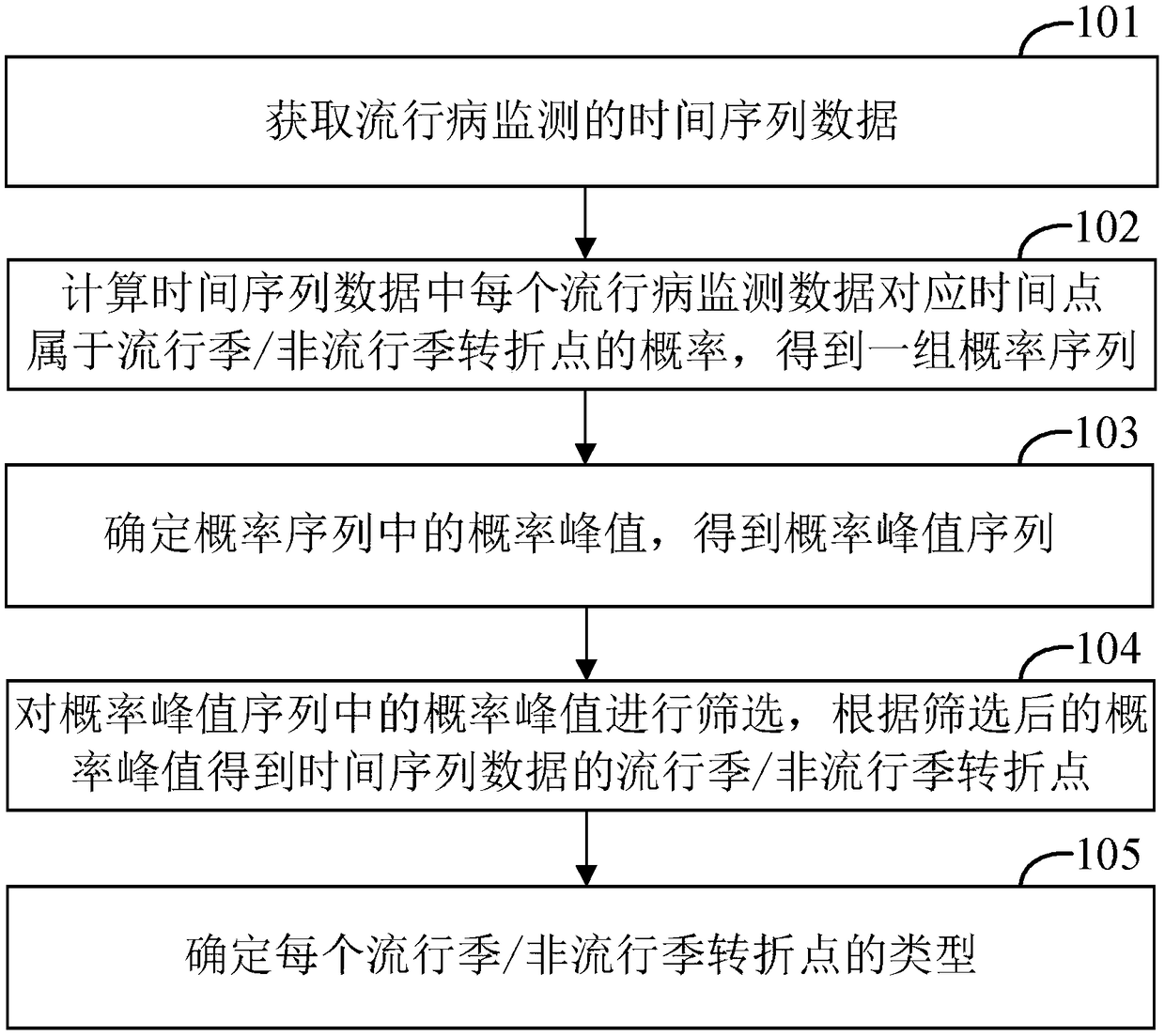
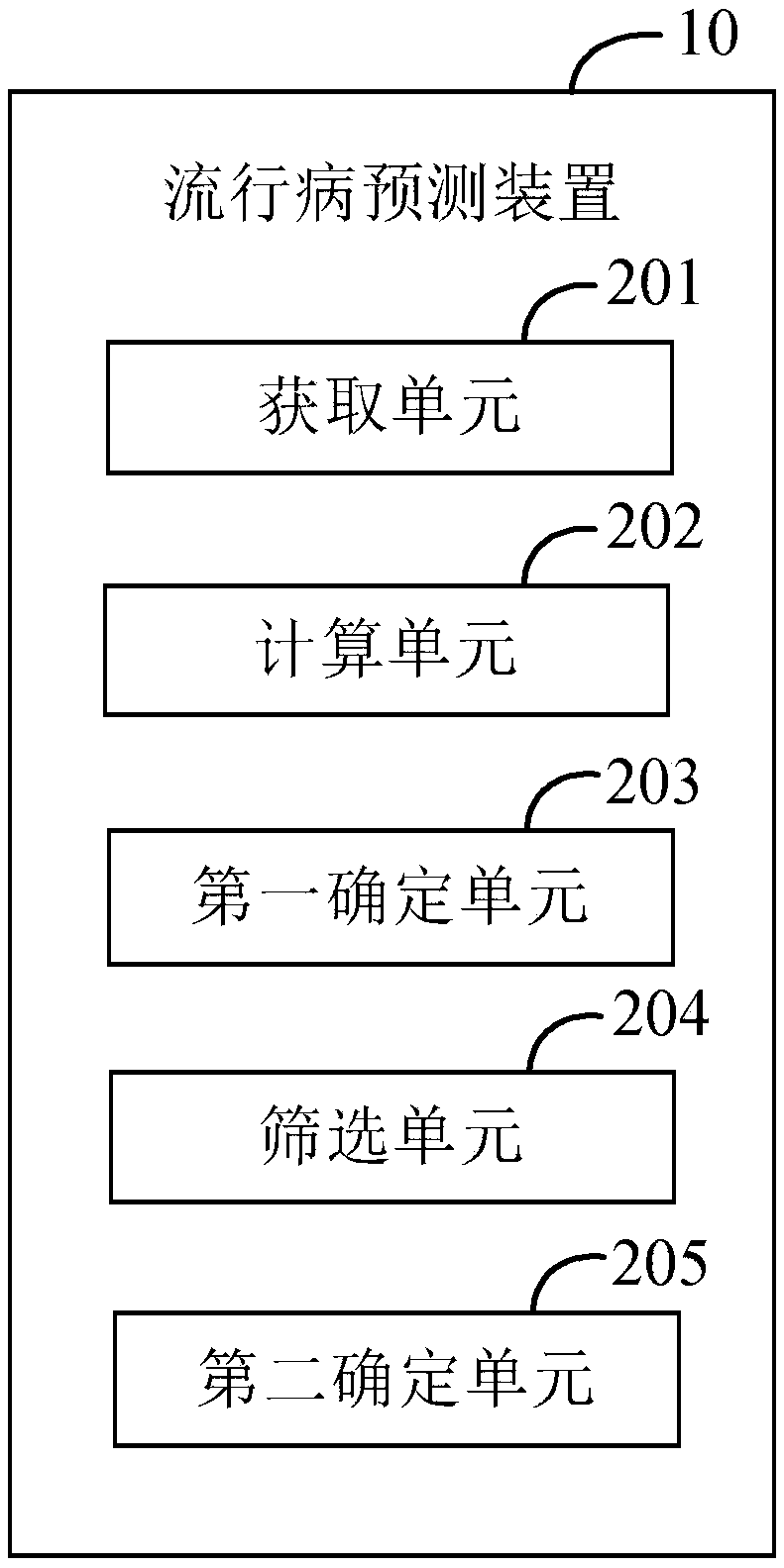
The invention provides an epidemiological prediction method, which comprises the steps of acquiring time sequence data of epidemiological monitoring; calculating the probability of time point corresponding to each epidemiological monitoring data in the time sequence data belonging to a popular season / a non-popular season turning point, and obtaining a set of probability sequences; determining theprobability peak value in the probability sequence, and obtaining a probability peak value sequence; screening the probability peak value in the probability peak value sequence, and obtaining the popular season / non-popular season turning point of the time sequence data according to the screened probability peak value; determining the types of each popular season / non-popular season turning point, wherein the types comprise a rising turning point and a descending turning point; the rising turning point is the starting point of the epidemiological popular season, and the descending turning pointis the ending point of the epidemiological popular season. The invention further provides a computer device and a readable storage medium. According to the invention, efficient and rapid epidemiological prediction can be realized.
Owner:PING AN TECH (SHENZHEN) CO LTD
Suspension array for detecting serotype enterobacter aerogenes typing and application
ActiveCN107586861AFill technology gapsShort detection timeMicrobiological testing/measurementAgainst vector-borne diseasesEpidemiological MonitoringBio plex
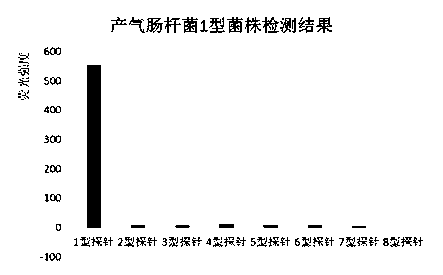
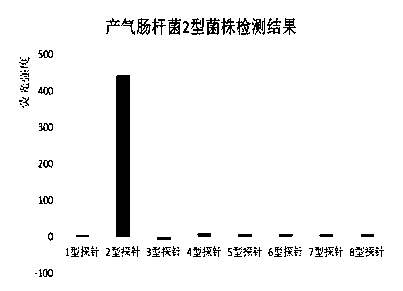
The invention provides a specific oligonucleotide sequence for detecting serotypes 1-8 of enterobacter aerogenes. The oligonucleotide contains: (1), a DNA fragment selected from wzy genes of the enterobacter aerogenes of types 1, 2, 3, 5, 6, 7 and 8; (2), a DNA fragment selected from orf8 genes of the enterobacter aerogenes of type 4. A suspension array detection system and method for the serotypes 1-8 of the enterobacter aerogenes are established through combination with a Bio-Plex 200 suspension array system of the Bio-Rad company, the blank of serological typing technology of the enterobacter aerogenes is filled up, and the invention has great significance in clinical detection and epidemiological monitoring of the important pathogenic bacterium.
Owner:NANKAI UNIV
Fluorescent quantitative PCR detection method for influenza viruses
PendingCN111270018AEfficient enrichmentOvercoming defects that affect detection sensitivityMicrobiological testing/measurementMicroorganism based processesEpidemiological MonitoringRNA extraction
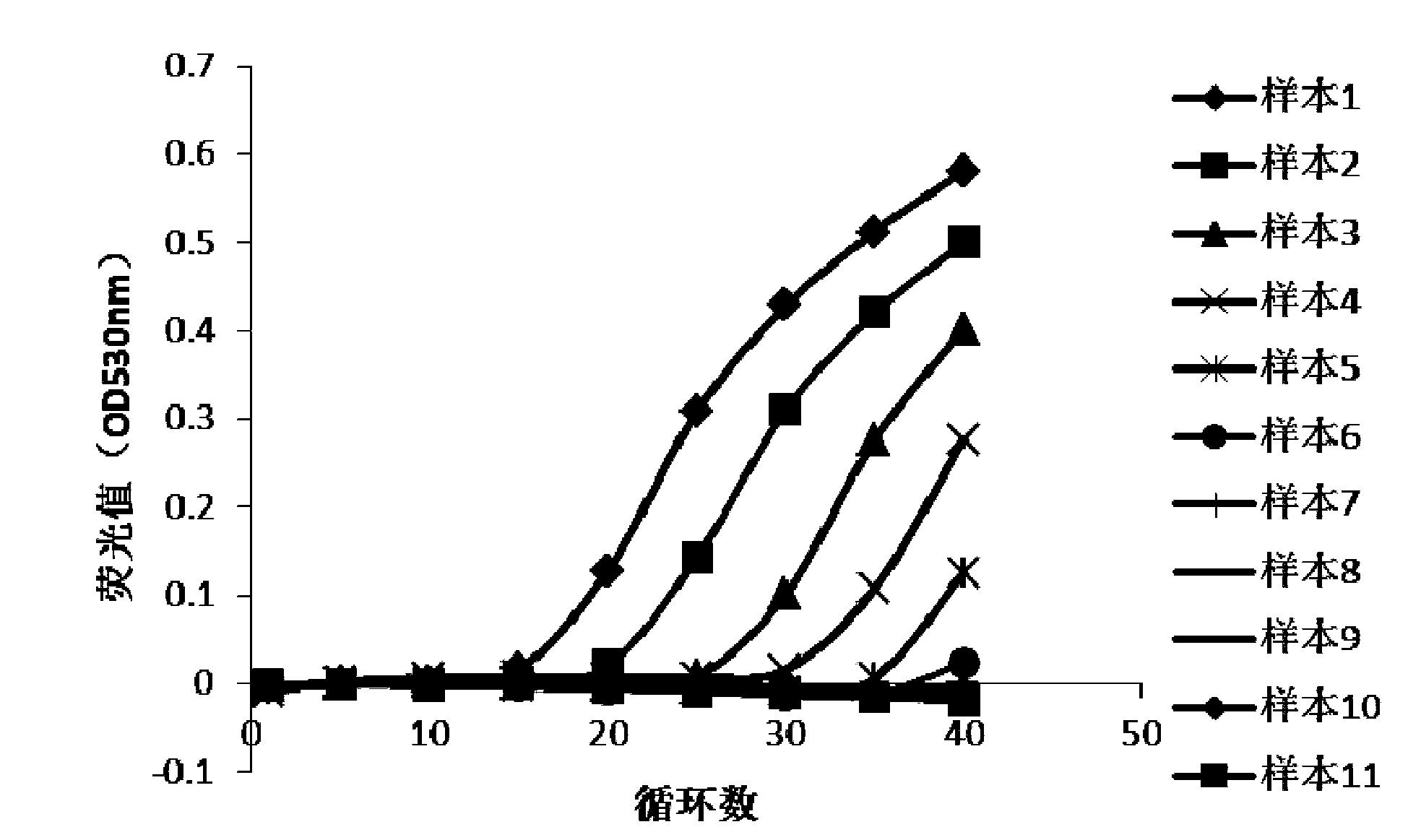
The invention discloses an fluorescent quantitative PCR detection method for influenza virus. The immunomagnetic beads and DEAE magnetic beads are mixed to obtain virus nucleic acid extraction magnetic beads; viral nucleic acid is extracted in a sample to be detected by using the obtained viral nucleic acid extraction magnetic beads; a high-resolution dissolution curve method combining a Taqman probe and a fluorescent quantitative PCR technology is adopted to perform fluorescent quantitative PCR amplification on extracted viral nucleic acid, so that typing detection of influenza viruses in a to-be-detected sample is realized. The method can be used for clinical rapid differential diagnosis, prevention and control during outbreak of epidemic situations, epidemiological monitoring of virusesand the like, and the method overcomes the defects that RNA extraction steps of tissue samples are various, sample virus RNA is caused, and the detection sensitivity is influenced.
Owner:GUANGXI BOTANICAL GARDEN OF MEDICINAL PLANTS
On-site rapid high-sensitivity detection kit and detection method of taura syndrome virus (TSV)
InactiveCN101643792AEasy to distinguishShort manufacturing timeMicrobiological testing/measurementMicroorganism based processesDiseaseTE buffer
The invention relates to an on-site rapid high-sensitivity detection kit and a detection method of taura syndrome virus (TSV). The on-site rapid high-sensitivity detection kit comprises a sampling tube, a rinsing tube filled with distilled water, a nucleic acid denaturation tube filled with TE buffer liquid, an amplification detection tube filled with amplification reaction liquid and nucleic acid dye, a negative control tube, a positive control tube, a FTA membrane, quick drying liquid, and the like. The detection method has higher specificity, sensitivity and convenience than the TR-PCR detection method and has the advantages of low cost, no toxicity, high safety, convenient use, more accurate and quicker detection and high sensitivity and can replace the prior related detection methods, such as a pathological section method, an electron-microscopic observation method, an antibody detection method and a TR-PCR detection method. The on-site rapid high-sensitivity detection kit and the detection method can be both used in an indoor laboratory and a production site in the field, have significance for strengthening the TSV epidemiological monitoring and the disease control and prevention and have high popularization and application value.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Preparation method of indirect ELISA detection kit based on theileria equi EMA1
InactiveCN108627649AEradicate hazardsPromote healthy developmentBiological testingEpidemiological MonitoringIndirect elisa
The invention relates to a preparation method of an indirect ELISA detection kit based on theileria equi EMA1. The preparation method comprises the preparation of theileria equi EMA1 and the establishment of a theileria equi disease rELISA detection method. The preparation method overcomes the defects of high cost, poor specificity and time consuming of a conventional detection kit, and establishes the theileria equi disease rELISA detection method which is suitable for the clinical diagnosis and epidemiological monitoring of theileria equi disease.
Owner:XINJIANG AGRI UNIV
Primer pair and primer probe composition used for identifying human adenovirus type 55, and application thereof
ActiveCN104073570AQuick checkReduce false positive resultsMicrobiological testing/measurementMicroorganism based processesAgricultural scienceEpidemiological Monitoring
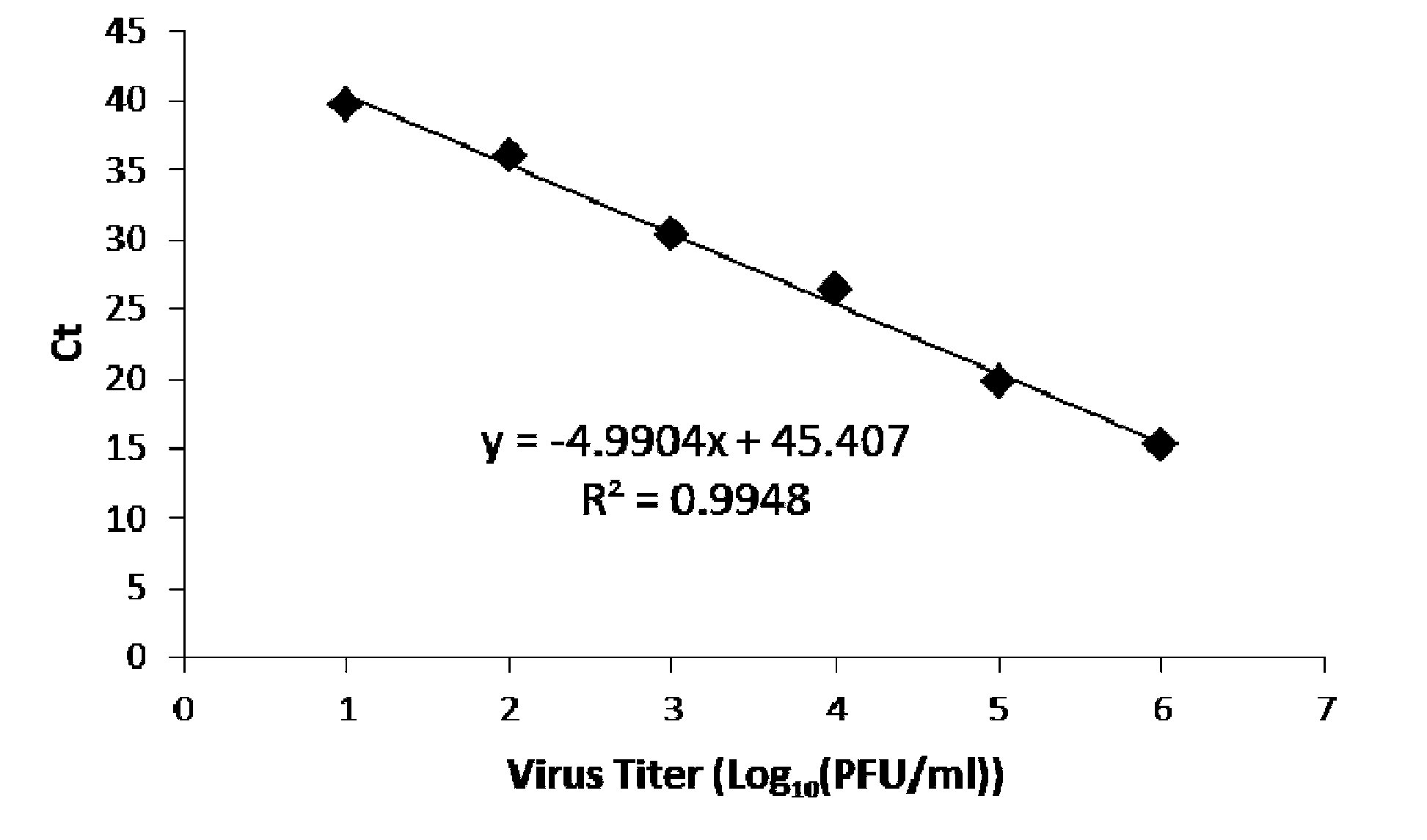
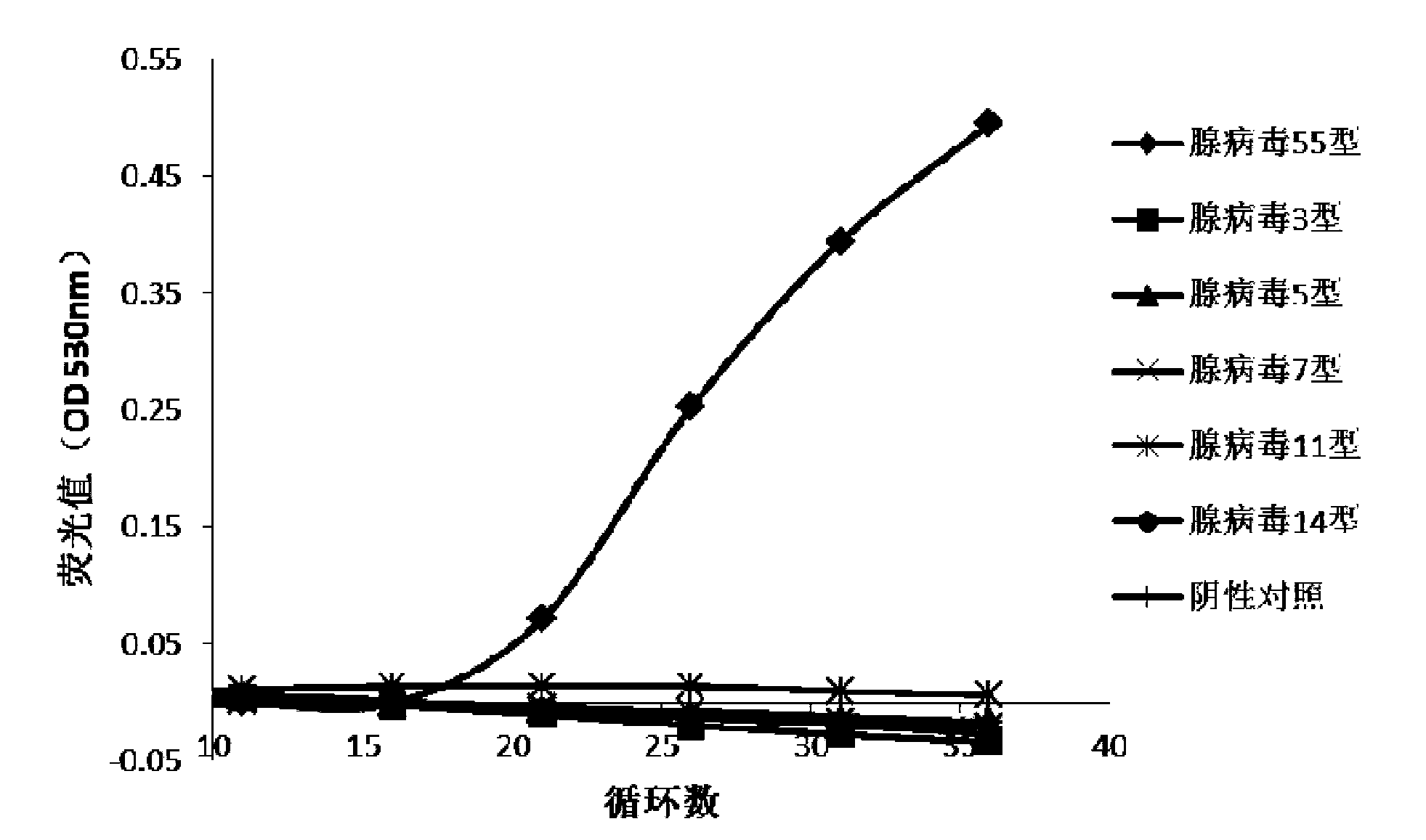
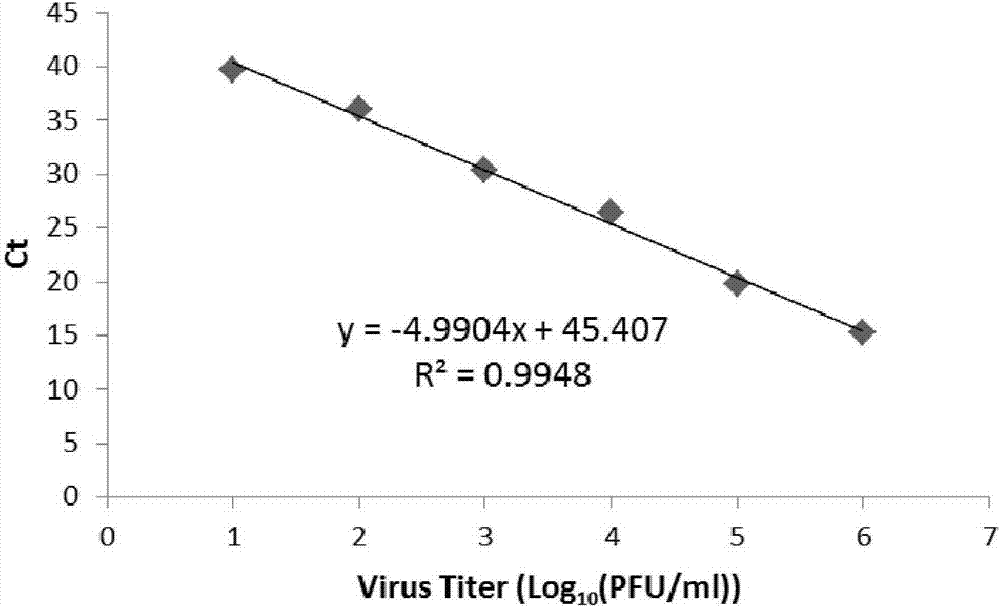
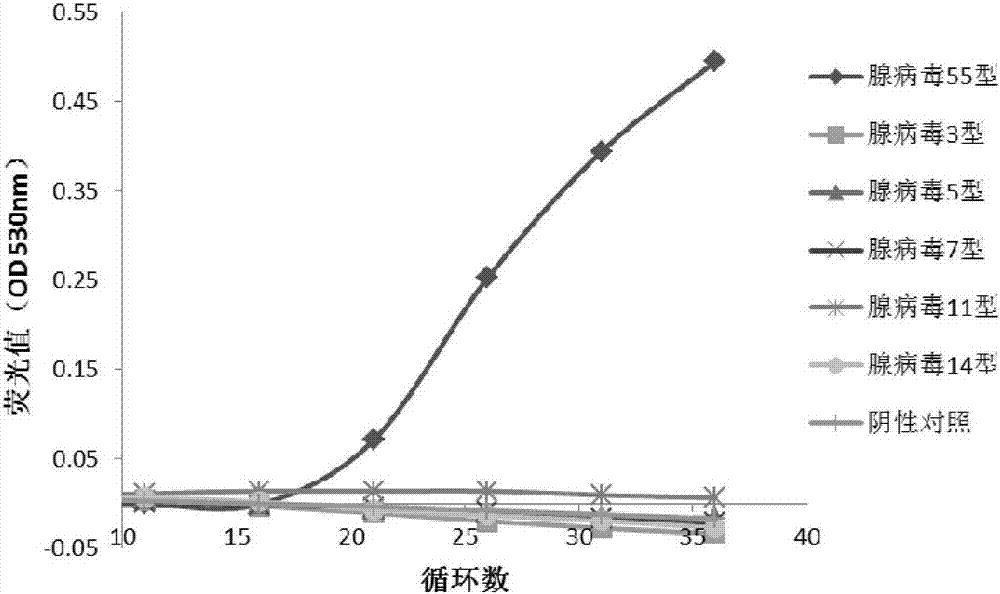
The invention discloses a primer pair and a primer probe composition used for identifying human adenovirus type 55 and application thereof. A kit for aided identification and / or quantitative determination of human adenovirus type 55 comprises the specific primer pair composed of a single-stranded DNA molecule as shown in a sequence 1 in a sequence table and a single-stranded DNA molecule as shown in a sequence 2 in the sequence table. The kit further comprises a specific probe which has a nucleotide sequence as shown in a sequence 3 in the sequence table or shown in a reverse complementary sequence of the sequence 3 in the sequence table. According to the invention, the disadvantages of a great consumption amount of samples, complex experimental operation, low detection sensitivity, poor specificity and the like of traditional detection technology are overcome, and the kit has high application specificity, shows detection sensitivity as same as that of a 0.008PFU / reaction system and is applicable to high flux screening, especially to scientific research, clinical practice, epidemiological monitoring of viruses, etc.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Method for preparing pure protein derivative of tubercle bacillus by using mycobacterium tuberculosis attenuated strain H37Ra
ActiveCN112813121AHarm reductionLower security level requirementsBacteriaMicroorganism based processesEpidemiological MonitoringImmunity response
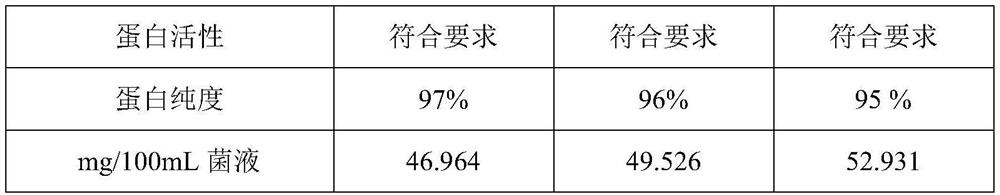
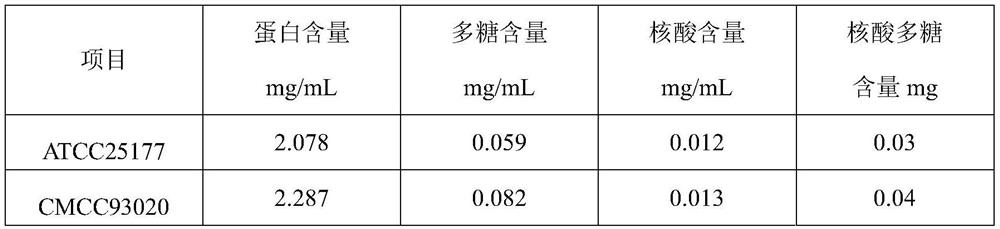
The invention discloses a method for preparing a pure protein derivative of tubercle bacillus by using a mycobacterium tuberculosis attenuated strain H37Ra, and belongs to the technical field of biological protein preparation. The mycobacterium tuberculosis attenuated strain CMCC93020 (H37Ra) is used as a strain, and a finished product of the pure protein derivative of the tubercle bacillus is obtained through amplification, inactivation, salting-out precipitation, degerming filtration, inspection, dilution and subpackaging. The defects in the prior art that strains have great harm to operators and the environment and have high requirements for production conditions (P3 production workshops) are overcome, and finally the protein which can be used for clinical diagnosis of tuberculosis, screening of bacillus calmette-guerin vaccine inoculation objects, monitoring of organism immune responses after bacillus calmette-guerin vaccine inoculation and epidemiological monitoring is obtained.
Owner:成都可恩生物科技有限公司
Production method of pure protein derivative of tuberculin
ActiveCN112899329AHarm reductionLower security level requirementsMicroorganism based processesPeptide preparation methodsEpidemiological MonitoringImmunity response
The invention discloses a production method of a pure protein derivative of tuberculin, and belongs to the technical field of biological protein preparation. An attenuated strain of mycobacterium tuberculosis ATCC25177 and / or CMCC93020 is used as a strain; and the finished product of the pure protein derivative of tuberculin is obtained through amplification, inactivation, salting-out sedimentation, degerming filtration, inspection, dilution and sub-packaging. The defect that strains have great harm to operators and the environment and have high requirements for production conditions (P3 production workshops) and the like in the prior art are overcome; and a protein, which can be used for clinical diagnosis of tuberculosis, screening of bacillus calmette-guerin vaccine inoculation objects, monitoring of organism immune response after bacillus calmette-guerin vaccine inoculation and epidemiological monitoring is finally obtained.
Owner:成都可恩生物科技有限公司
Colloidal gold test paper for detecting IBV, and preparation method thereof
The invention discloses colloidal gold test paper for detecting IBV. The colloidal gold test paper comprises a support plate, a coating film, a sample pad, a gold-labeled antibody pad, water absorbingpaper, a detection line and a quality control line, and is characterized in that the gold-labeled antibody pad is labeled with an anti-IBV IgG antibody, the detection line is coated with an anti-IBVN protein monoclonal antibody, and the quality control line is coated with a goat anti-mouse IgG antibody. The invention also discloses an index method of the colloidal gold test paper for detecting IBV. The test paper prepared by the invention is based on the principle of a double-antibody sandwich ELISA antigen detection method, is used for detecting IBV antigens so as to intuitively, quickly, simply and conveniently detect virus infection and achieve good specificity and the purpose of accurate and quick detection, can provide technical support for immune monitoring and epidemiological monitoring research of avian infectious bronchitis, and has important significance in prevention and control of avian diseases.
Owner:ZHENGZHOU UNIV
Field fast high-sensitivity detection kit and detection method for prawn hepatopancreatic parvovirus
ActiveCN101838707AQuick checkShort manufacturing timeMicrobiological testing/measurementMicroorganism based processesDiseaseTE buffer
The invention relates to a field fast high-sensitivity detection kit and a detection method for prawn hepatopancreatic parvovirus. The detection kit comprises a sampling pipe, a rinsing pipe filled with distilled water, a nucleic acid denaturation pipe filled with a TE buffer solution, an amplification detection pipe filled with amplification reactant liquid and nucleic acid dyes, a negative control pipe, a positive control pipe, an FTA diaphragm, quick drying liquid thereof and the like. The detection method has higher specificity, sensitivity and convenience than that of the PCR detection method, has the advantages of low cost, convenient operation, more accurate and faster detection and extremely high sensitivity, is safe to both human beings and the environment, and can replace the existing relevant detection methods such as the pathological section method, the electron microscope method and the PCR detection method. The detection kit and the detection method can be used in the indoor laboratory or the outdoor production field, are of great significance for enhancing the epidemiological monitoring and the disease control on prawn hepatopancreatic parvovirus, and have higher popularization and application values.
Owner:北海南方海洋科技开发有限公司
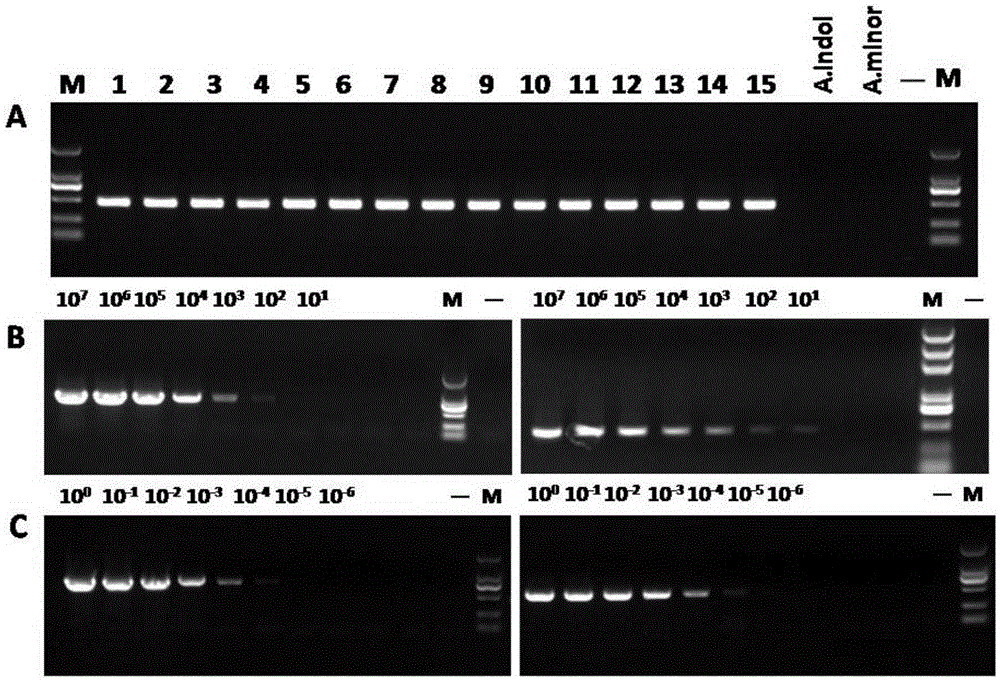
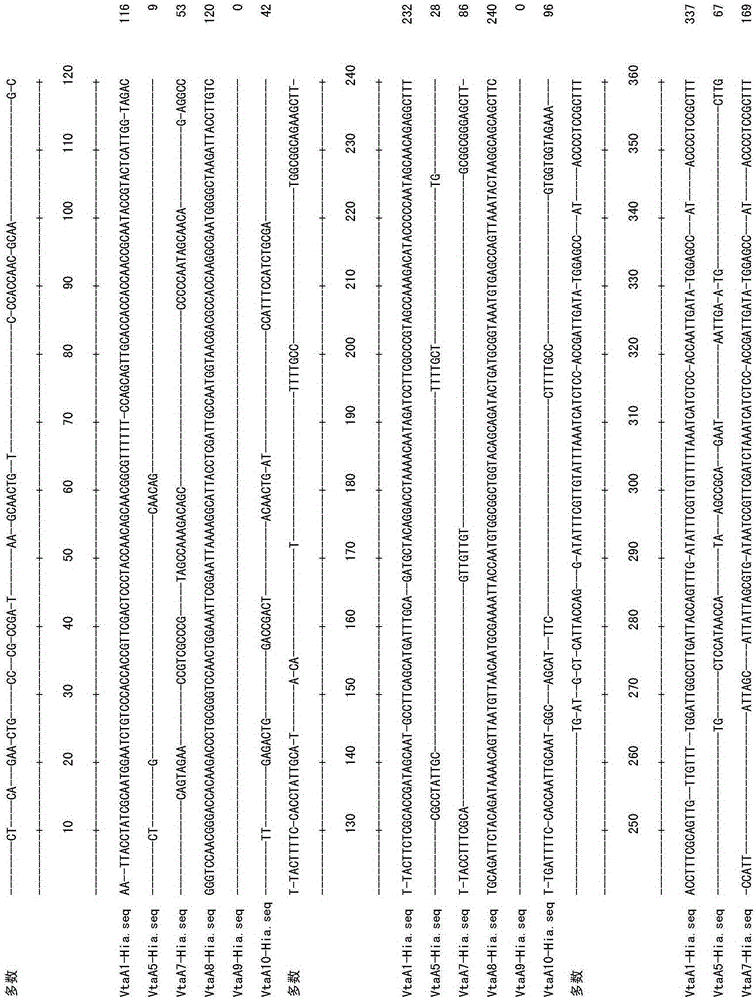
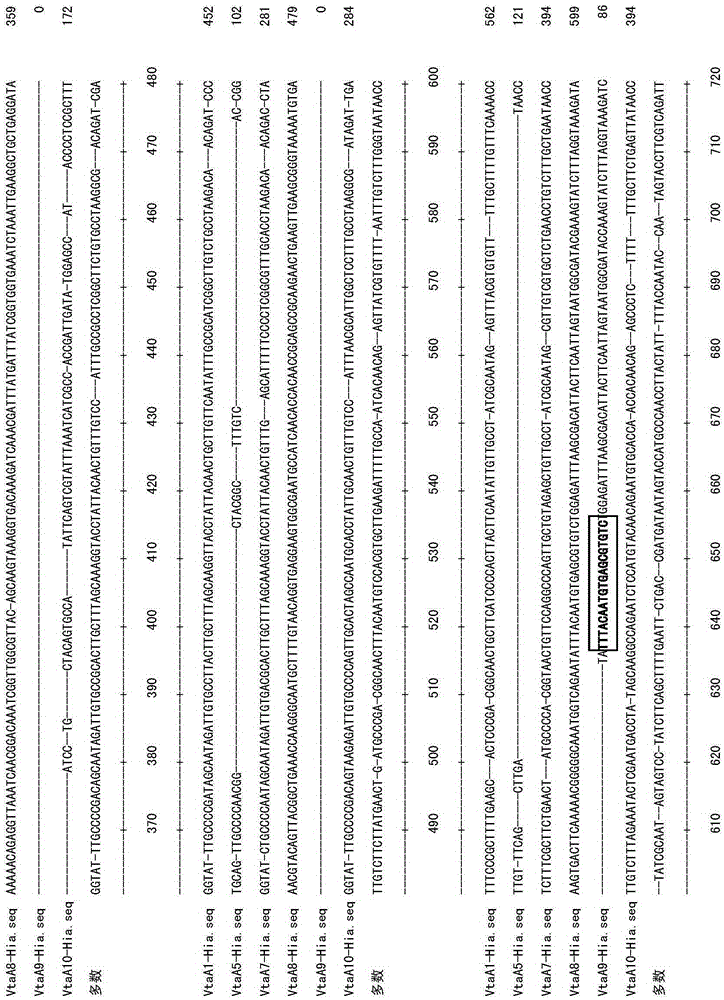
Primer group using vtaA9 gene as target spot and application of primer group to identification and diagnosis of haemophilus parasuis
InactiveCN103789422AImprove accuracyBroad laboratory application prospectsMicrobiological testing/measurementDNA/RNA fragmentationEpidemiological MonitoringHaemophilus
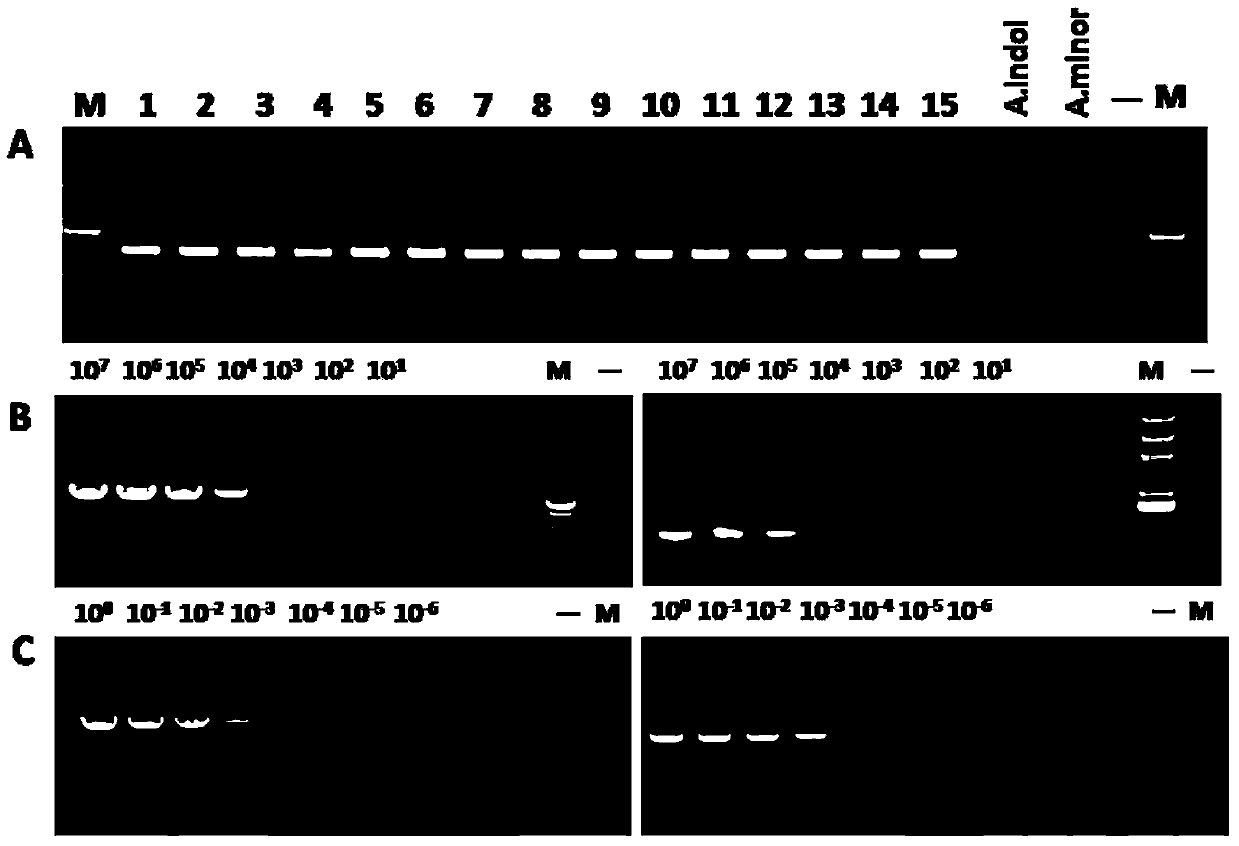
The invention discloses a primer group using a vtaA9 gene as a target spot and application of the primer group to identification and diagnosis of haemophilus parasuis. Researches find that a PCR (Polymerase Chain Reaction) method using the vtaA9 gene as the target spot is capable of accurately distinguishing different pathogenicities, and can simultaneously settle in swine upper respiratory tract H.parasuis and similar strains A.indolicus, A.minor and A.porcinus thereof. In addition, based on the vtaA9 gene sequence, the invention designs and synthesizes a specific primer, wherein nucleotide sequences of the primer are shown as SEQ ID NO.1 and SEQ ID NO.2. Specificity experiments indicate that according to the method disclosed by the invention, the H.parasuis and the A.indolicus as well as the A.minor and the A.porcinus can be completely distinguished. Sensitivity experiments indicate that by using the method disclosed by the invention, genomes DNA of not less than 20CFU / ml and 5pg / mu l can be detected. In addition, a positive result of the PCR method using the vtaA9 as the target spot for a clinical sample is higher than that of isolated culture of a strain and a classic 16S rRNA (ribosomal ribonucleic acid) PCR method. The invention provides a novel method for differential diagnosis of haemophilus parasuis and epidemiological monitoring.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Molecular detection method for rapid detection of Ile-1781-Leu mutation of anti-fenoxaprop-P-ethyl Beckmannia syzigachne
InactiveCN103820551AHigh sensitivityStrong specificityMicrobiological testing/measurementEpidemiological MonitoringFluorescence
The invention discloses a molecular detection method for rapid identification of genotypes of anti-fenoxaprop-P-ethyl Beckmannia syzigachne containing Ile-1781-Leu mutation based on LAMP technology. The detection method can be used for early warning and epidemiological monitoring of Beckmannia syzigachne containing the Ile-1781-Leu mutation. The detection method comprises altogether three main steps: (1) respectively extracting genomic DNA of a sample to be detected; (2) designing specific primers and carrying out LAMP isothermal amplification; and (3) adding the fluorescent dye SYBR Green I into an LAMP amplification product and observing color changes, or carrying out separation through agarose gel electrophoresis with 3.0% agarose and observing amplification results, wherein whether there is a population of Beckmannia syzigachne containing the Ile-1781-Leu mutation is determined based on the results, a population of Beckmannia syzigachne is determined to be a population containing the Ile-1781-Leu mutation if the LAMP amplification product is yellow or electrophoretogram is in the form of a scalariform strip, and a population of Beckmannia syzigachne is determined to be a population not containing the Ile-1781-Leu mutation if the LAMP amplification product is orange or electrophoretogram has no strip amplification. According to the invention, isothermal amplification is realized, expensive instruments and equipment and tedious electrophoresis process are not needed, and detection time and cost are substantially reduced. Compared with common PCR, the detection method provided by the invention has the characteristics of high sensitivity, strong specificity, high detection accuracy, etc.
Owner:NANJING AGRICULTURAL UNIVERSITY
Fluorescence quantitative PCR method for fast detecting Campylobacter jejuni macrolide drug resistant mutational site
ActiveCN101348831BHigh amplification efficiencyAccurate detectionMicrobiological testing/measurementFluorescence/phosphorescenceBiotechnologyEpidemiological Monitoring
The invention belongs to the biotechnical field, relating to a method for detecting drug-resistant related gene mutation of campylobacter jejuni macrolide drugs. The method makes use of a realtime fluorescence quantitative PCR TaqMan probe technology to design a specific primer and a probe according to a nucleic acid sequence of a V zone inside a campylobacter jejuni macrolide drug-resistant mutation zone 23S rDNA; moreover, through optimizing a TaqMan realtime fluorescence quantitative PCR reaction system and conditions, related drug-resistant mutational sites of target genes are accurately detected. The method can realize quick, accurate and simultaneous detection of two main drug-resistant mutational sites of campylobacter jejuni macrolide type, and has better specificity and higher sensitivity than a related molecular biology detection method reported at present; moreover, the method has simple operation, high accuracy and quick speed compared with the prior sequencing and drug sensitive experimental method. The method provides an effective technical means for molecular epidemiological monitoring of macrolide campylobacter jejuni resistance.
Owner:HUAZHONG AGRI UNIV
On-site rapid high-sensitivity detection kit and detection method of prawn yellow head virus
InactiveCN101921872AShorten the timeShorten detection timeMicrobiological testing/measurementDiseaseTE buffer
The invention relates to an on-site rapid high-sensitivity detection kit and a detection method of prawn yellow head virus. The on-site rapid high-sensitivity detection kit comprises a sampling tube, a rinsing tube filled with distilled water, a nucleic acid denaturation tube filled with TE buffer liquid, an amplification detection tube filled with amplification reaction liquid and nucleic acid dye, a negative control tube, a positive control tube, an FTA membrane, quick drying liquid, and the like. Compared with an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection method, the detection method has higher specificity, sensitivity and convenience, and has the advantages of low cost, convenient use, more accurate and quicker detection, high sensitivity, and high safety to human and environment, and can replace the traditional related detection methods, such as an electron-microscopic observation method, an RT-PCR detection method, and the like. The on-site rapid high-sensitivity detection kit and the detection method can be used in both an indoor laboratory and a production site in the field, have great significance for strengthening the prawn yellow head virus epidemiological monitoring, prawn culturing water monitoring and disease control and prevention, and have high popularization and application value.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Method for detecting ten kinds of trimethoprim drug resistance genes and kits used
InactiveCN108642169BHigh degree of integrationHigh sensitivityMicrobiological testing/measurementResistant genesEpidemiological Monitoring
The invention discloses a kit for detecting drug resistance genes of ten trimethoprim drugs. The kit comprises an amplification primer combination for carrying out specific amplification on the drug resistance genes of the ten trimethoprim drugs and a specific probe for detecting on the drug resistance genes of the ten trimethoprim drugs. The invention further discloses a method for detecting thedrug resistance genes of the ten trimethoprim drugs by using the kit. The method for detecting the drug resistance genes of the trimethoprim drugs and the kit thereof have the characteristics of highintegration degree, high sensitivity, good specificity, stable and reliable detection results and low cost, can be widely used for distribution and epidemiological monitoring of the drug resistance genes of the trimethoprim drugs, and provides an effective tool for rational drug use and effective reduction and delay of drug resistance.
Owner:ZHEJIANG UNIV
Field fast high-sensitivity detection kit and detection method for prawn hepatopancreatic parvovirus
ActiveCN101838707BQuick checkShort manufacturing timeMicrobiological testing/measurementMicroorganism based processesDiseaseTE buffer
The invention relates to a field fast high-sensitivity detection kit and a detection method for prawn hepatopancreatic parvovirus. The detection kit comprises a sampling pipe, a rinsing pipe filled with distilled water, a nucleic acid denaturation pipe filled with a TE buffer solution, an amplification detection pipe filled with amplification reactant liquid and nucleic acid dyes, a negative control pipe, a positive control pipe, an FTA diaphragm, quick drying liquid thereof and the like. The detection method has higher specificity, sensitivity and convenience than that of the PCR detection method, has the advantages of low cost, convenient operation, more accurate and faster detection and extremely high sensitivity, is safe to both human beings and the environment, and can replace the existing relevant detection methods such as the pathological section method, the electron microscope method and the PCR detection method. The detection kit and the detection method can be used in the indoor laboratory or the outdoor production field, are of great significance for enhancing the epidemiological monitoring and the disease control on prawn hepatopancreatic parvovirus, and have higher popularization and application values.
Owner:北海南方海洋科技开发有限公司
A primer set targeting the vtaa9 gene and its application in the identification and diagnosis of Haemophilus parasuis
InactiveCN103789422BImprove accuracyBroad laboratory application prospectsMicrobiological testing/measurementDNA/RNA fragmentationEpidemiological MonitoringNucleotide
The invention discloses a primer group using a vtaA9 gene as a target spot and application of the primer group to identification and diagnosis of haemophilus parasuis. Researches find that a PCR (Polymerase Chain Reaction) method using the vtaA9 gene as the target spot is capable of accurately distinguishing different pathogenicities, and can simultaneously settle in swine upper respiratory tract H.parasuis and similar strains A.indolicus, A.minor and A.porcinus thereof. In addition, based on the vtaA9 gene sequence, the invention designs and synthesizes a specific primer, wherein nucleotide sequences of the primer are shown as SEQ ID NO.1 and SEQ ID NO.2. Specificity experiments indicate that according to the method disclosed by the invention, the H.parasuis and the A.indolicus as well as the A.minor and the A.porcinus can be completely distinguished. Sensitivity experiments indicate that by using the method disclosed by the invention, genomes DNA of not less than 20CFU / ml and 5pg / mu l can be detected. In addition, a positive result of the PCR method using the vtaA9 as the target spot for a clinical sample is higher than that of isolated culture of a strain and a classic 16S rRNA (ribosomal ribonucleic acid) PCR method. The invention provides a novel method for differential diagnosis of haemophilus parasuis and epidemiological monitoring.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
The application of Mycobacterium tuberculosis antigenic protein rv2201 and its T-cell epitope peptide
ActiveCN106248936BReduce false positivesAntibacterial agentsBacterial antigen ingredientsMycobacterium InfectionsAntituberculous drugs
The invention relates to application of a mycobacterium tuberculosis antigen protein Rv2201 and a T cell epitope peptide thereof to preparation of a tuberculosis detection reagent, vaccine and medicament. Amino acid sequences of the mycobacterium tuberculosis antigen protein Rv2201 and the T cell epitope peptide thereof are shown as SEQ ID NO:1-3. The mycobacterium tuberculosis antigen protein Rv2201 and the T cell epitope peptide thereof are adopted for immunoreaction of specific T cells and B cells caused by mycobacterium tuberculosis infection as irritants, and compared with conventional adoption of a complete antigen, adoption of the mycobacterium tuberculosis antigen protein Rv2201 and the T cell epitope peptide thereof has the advantage that a false positive result caused by impurity of the antigen can be reduced. The detection reagent prepared from the antigen protein Rv2201 and the epitope peptide thereof can be widely applied to the related fields of auxiliary diagnosis of tuberculosis, epidemiological monitoring and the like, and the tuberculosis vaccine and anti-tuberculosis medicament prepared from the antigen protein Rv2201 and the epitope peptide thereof can be used for prevention and treatment of tuberculosis.
Owner:ICDC CHINA CDC
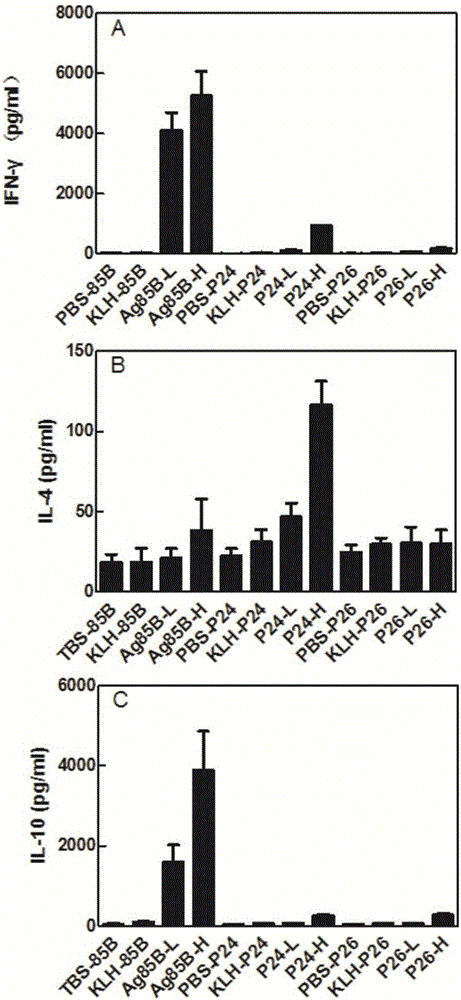
Mycobacterium tuberculosis antigen protein Rv0585c and application of T cell epitope peptide thereof
ActiveCN106397553AReduce false positivesStrong immune responseAntibacterial agentsBacterial antigen ingredientsMycobacterium InfectionsAntituberculous drugs
The invention relates to mycobacterium tuberculosis antigen protein Rv0585c and application of a T cell epitope peptide thereof in preparing tuberculosis detection reagents, vaccines and medicines. The epitope peptide is selected from P24, P26 and P31 which have amino acid sequences shown as SEQ ID NO:1, SEQ ID NO:2 and SEQ ID NO:3 respectively. A mycobacterium tuberculosis protein antigen Rv0585c and the T cell epitope peptide thereof serve as irritants and are used for specific T cell and B cell immunoreaction caused by mycobacterium tuberculosis infection; compared with an existing adopted complete antigen, the false positive result caused by antigen impurity can be reduced. The detection reagents prepared from the protein antigen Rv0585c and the epitope peptide of the protein antigen Rv0585c can be widely used for auxiliary diagnosis, epidemiological monitoring and other related fields of tuberculosis. Tuberculosis vaccines and antituberculosis medicines prepared from the protein antigen Rv0585c and the epitope peptide of the protein antigen Rv0585c can be used for preventing and treating tuberculosis.
Owner:ICDC CHINA CDC
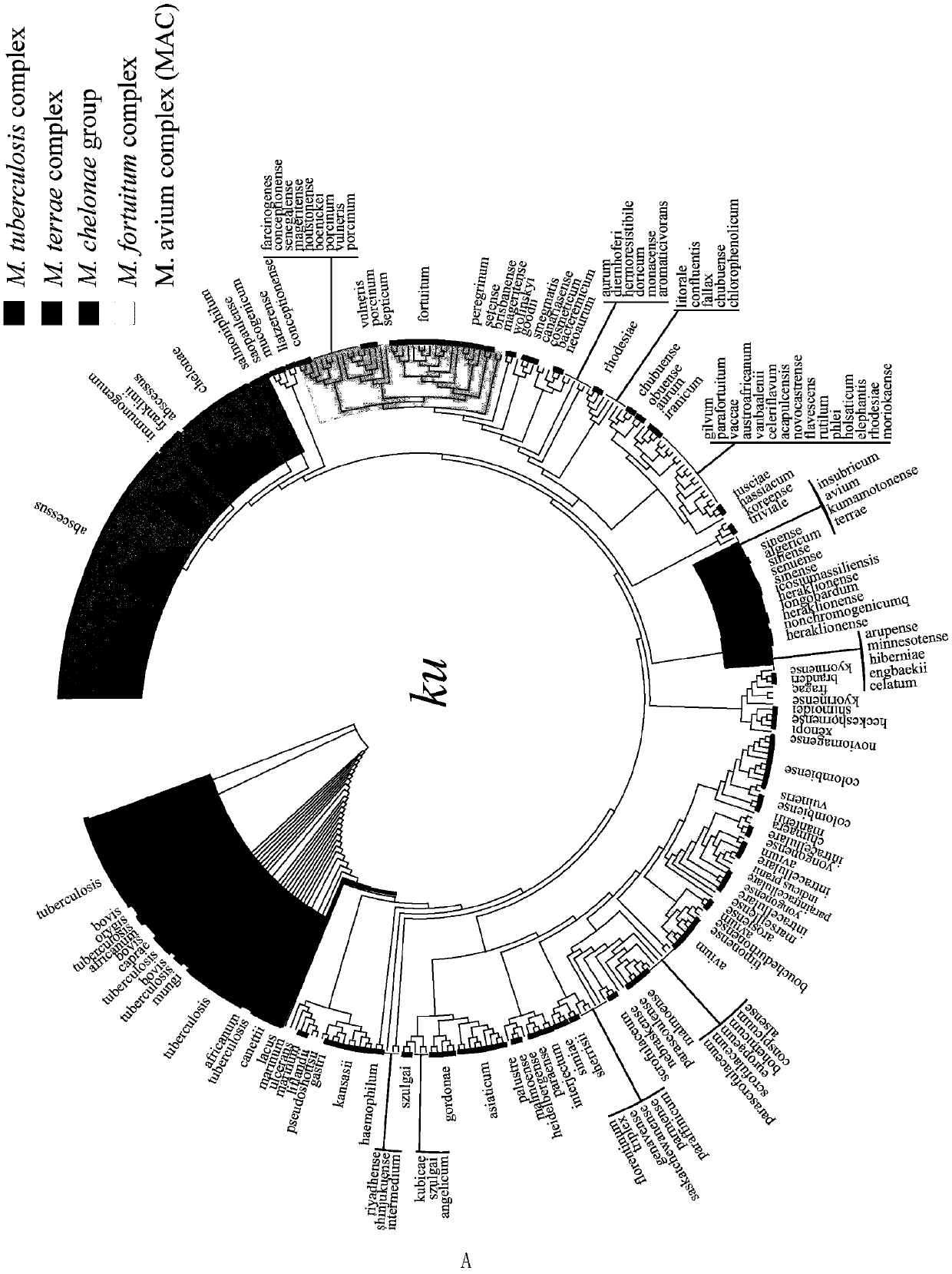
Application of mycobacteria Ku protein
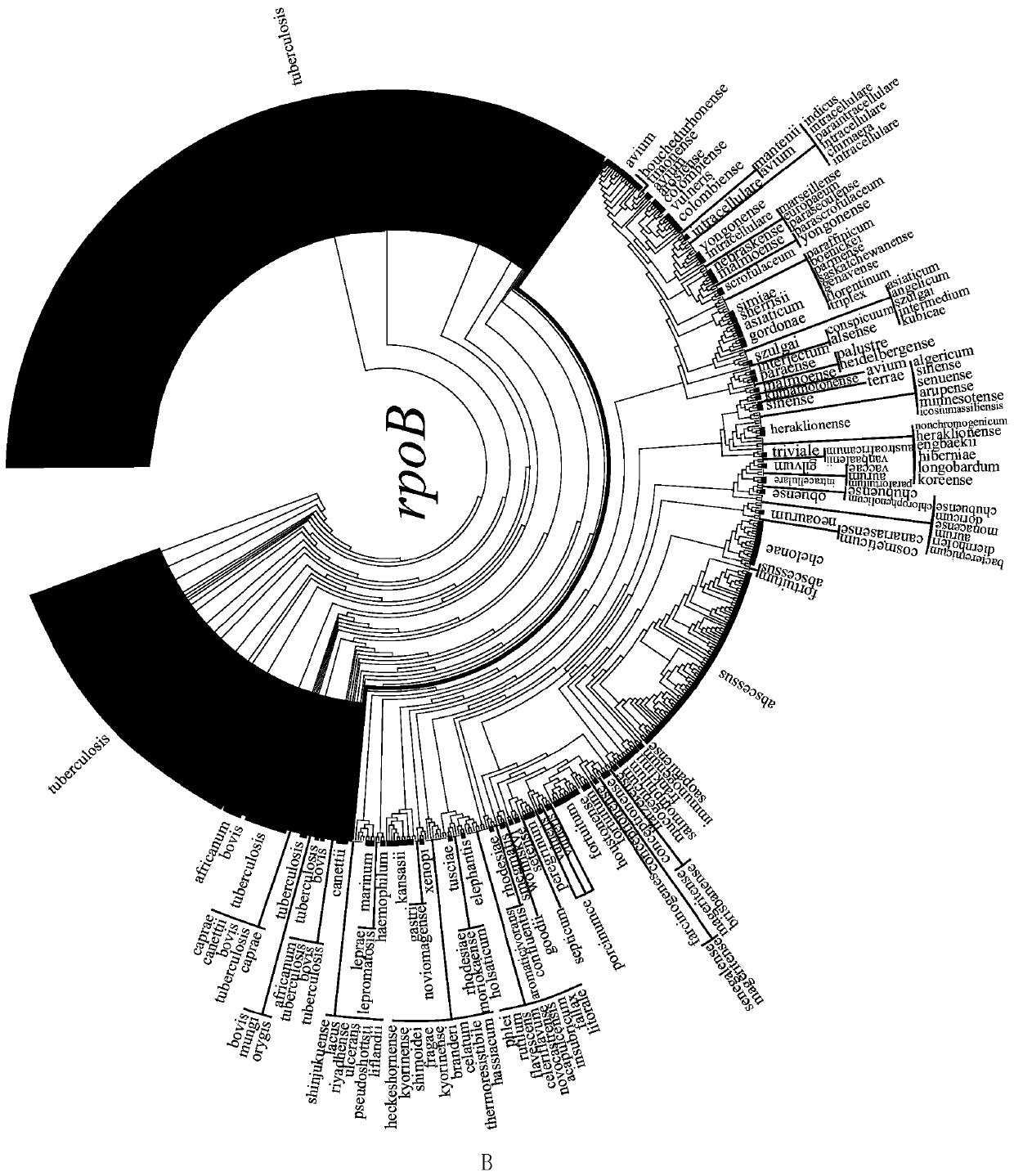
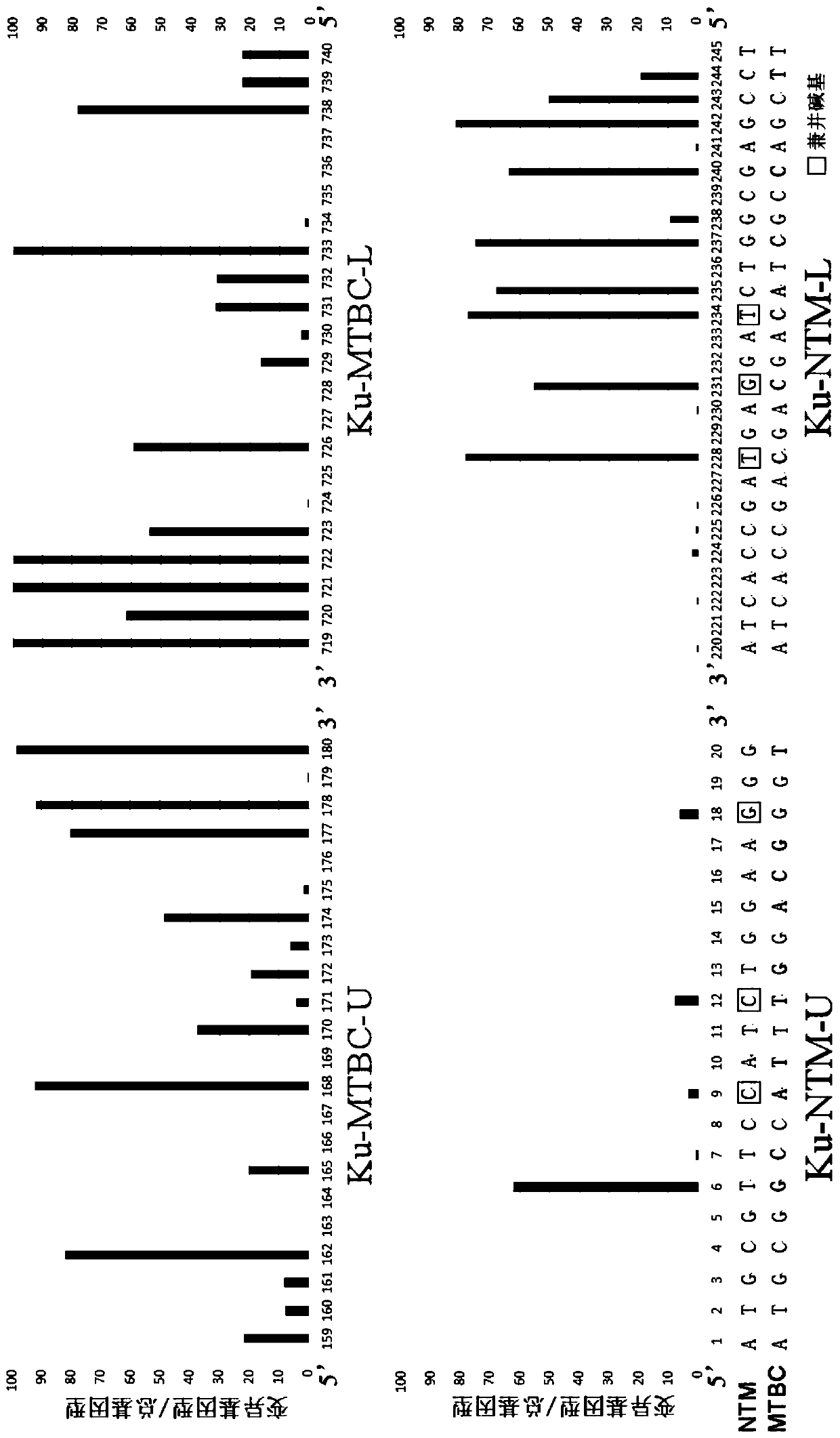
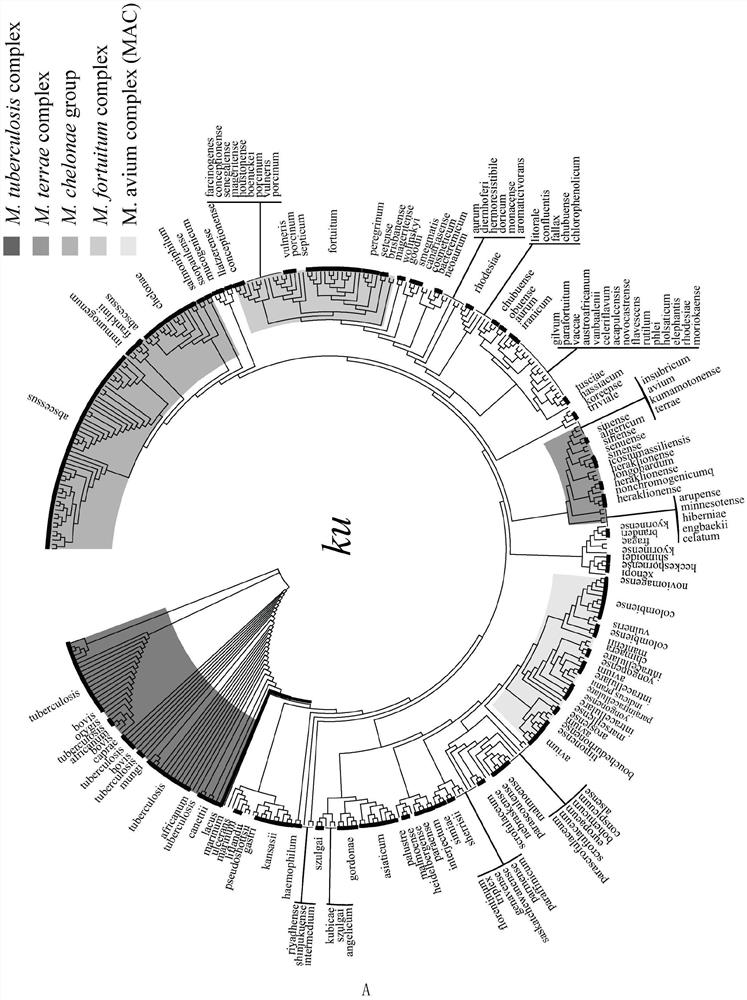
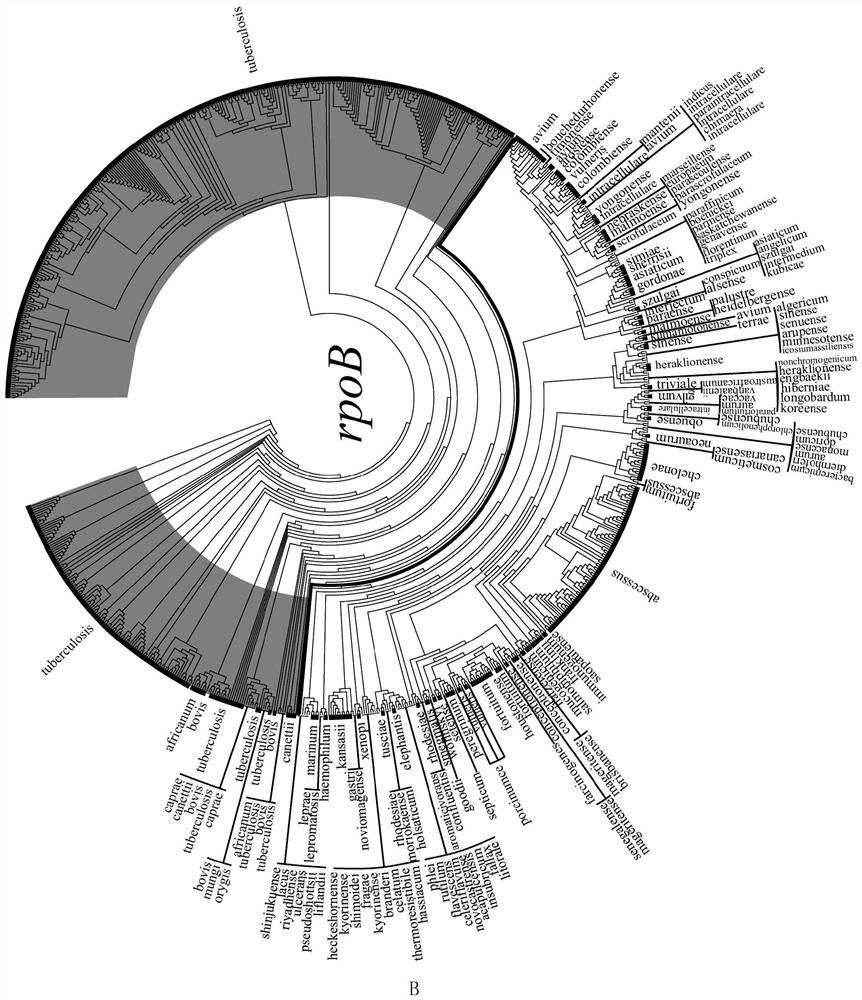
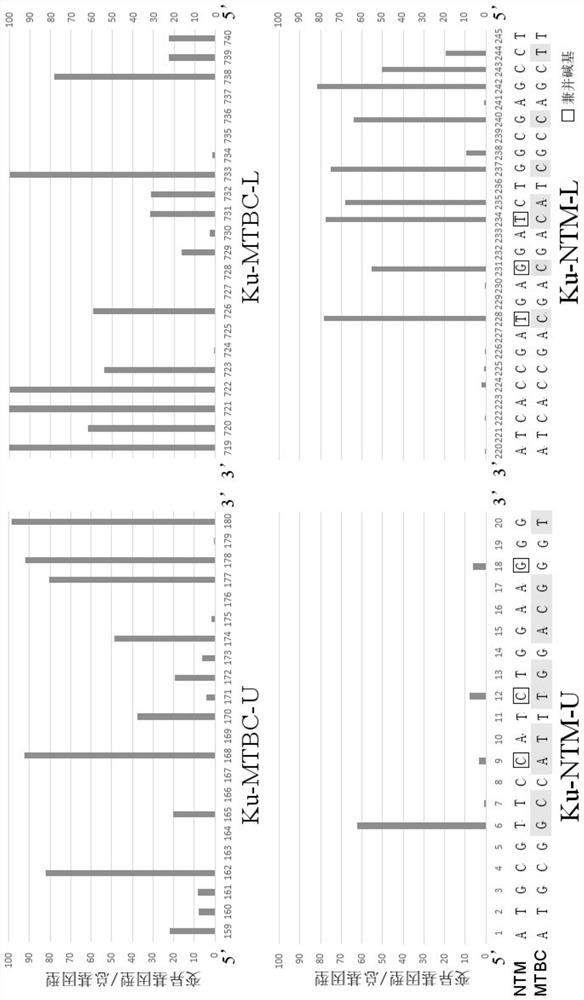
ActiveCN109975543AGuaranteed specificityAntibacterial agentsBacterial antigen ingredientsEpidemiological MonitoringAssay
The invention provides the application of mycobacteria Ku protein. The invention provides a novel marker which belongs to ku gene / protein of an NHEJ system and can be used for the identification of mycobacteria and the differential identification of MTBC and NTM. The prevalence of the ku gene in mycobacteria and the rarity in prokaryotic cells determine the applicability and high specificity of adetection technology based on the marker to the entire mycobacterium. Based on the ku gene / protein, rapid identification methods such as PCR, RT- PCR, LAMP-PCR and enzyme-linked immunosorbent assay (ELISA) and immunocolloidal gold can be established, and corresponding reagents can be developed. A detection reagent based on the invention can be widely applied to related fields such as auxiliary diagnosis of tuberculosis and epidemiological monitoring.
Owner:ICDC CHINA CDC
Epidemic prediction method, computer device, and computer-readable storage medium
ActiveCN108630321BEfficient forecastingAvoid spending a lot of timeMedical data miningEpidemiological alert systemsEpidemiological MonitoringAlgorithm
Owner:PING AN TECH (SHENZHEN) CO LTD
Primer set for rapid detection of gyra gene mutation in Campylobacter jejuni and its application
ActiveCN103525929BStrong specificityImprove accuracyMicrobiological testing/measurementMicroorganism based processesEpidemiological MonitoringSingle strand
The invention discloses a primer group for quickly detecting the gyrA gene mutation site of campylobacter jejuni and an application thereof. The primer group consists of a specific primer pair and a sequencing primer, wherein the specific primer pair consists of a single-strand DNA molecule A shown by the sequence 1 and a single-strand DNA molecule B shown by the sequence 2, and the sequencing primer is a single-strand DNA molecule C shown by the sequence 3. By adopting the campylobacter jejuni to be detected as a template and adopting the specific primer pair, a gyrA gene segment including a 257 mutation site is obtained through a PCR (polymerase chain reaction), and a DNA single strand can be obtained through single-strand separation and purification of PCR products and is directly put into a pyrosequencing apparatus for sequencing. By adopting the primer group disclosed by the invention, the C257T mutation of campylobacter jejuni can be identified so as to determine whether the campylobacter jejuni to be detected tolerates quinolone antibiotics at a high level. A powerful technical means is provided for the detection of anti-quinolone campylobacter jejuni as well as molecular epidemiological monitoring.
Owner:CHINA AGRI UNIV
Mycobacterium tuberculosis antigen protein Rv1808 and application of epitope peptide thereof
InactiveCN113173979AAntibacterial agentsBacterial antigen ingredientsEpidemiological MonitoringAntituberculosis drug
The invention belongs to the technical field of biology, and particularly relates to a mycobacterium tuberculosis antigen protein Rv1808 and an application of an epitope peptide thereof. A detection reagent prepared from the Rv1808 protein and the epitope peptide thereof can be widely applied to the related fields of auxiliary diagnosis of tuberculosis, epidemiological monitoring and the like, and tuberculosis vaccines and antituberculosis drugs prepared from the Rv1808 protein and the epitope peptide thereof can be applied to prevention and treatment of tuberculosis.
Owner:HELIXGEN GUANGZHOU
The application of Mycobacterium tuberculosis antigenic protein rv0585c and its T-cell epitope peptide
ActiveCN106397553BReduce false positivesStrong immune responseAntibacterial agentsBacterial antigen ingredientsEpidemiological MonitoringTreating tuberculosis
The invention relates to mycobacterium tuberculosis antigen protein Rv0585c and application of a T cell epitope peptide thereof in preparing tuberculosis detection reagents, vaccines and medicines. The epitope peptide is selected from P24, P26 and P31 which have amino acid sequences shown as SEQ ID NO:1, SEQ ID NO:2 and SEQ ID NO:3 respectively. A mycobacterium tuberculosis protein antigen Rv0585c and the T cell epitope peptide thereof serve as irritants and are used for specific T cell and B cell immunoreaction caused by mycobacterium tuberculosis infection; compared with an existing adopted complete antigen, the false positive result caused by antigen impurity can be reduced. The detection reagents prepared from the protein antigen Rv0585c and the epitope peptide of the protein antigen Rv0585c can be widely used for auxiliary diagnosis, epidemiological monitoring and other related fields of tuberculosis. Tuberculosis vaccines and antituberculosis medicines prepared from the protein antigen Rv0585c and the epitope peptide of the protein antigen Rv0585c can be used for preventing and treating tuberculosis.
Owner:ICDC CHINA CDC
On-site rapid high-sensitivity detection kit and detection method of taura syndrome virus (TSV)
InactiveCN101643792BEasy to distinguishShort manufacturing timeMicrobiological testing/measurementMicroorganism based processesDiseaseEpidemiological Monitoring
The invention relates to an on-site rapid high-sensitivity detection kit and a detection method of taura syndrome virus (TSV). The on-site rapid high-sensitivity detection kit comprises a sampling tube, a rinsing tube filled with distilled water, a nucleic acid denaturation tube filled with TE buffer liquid, an amplification detection tube filled with amplification reaction liquid and nucleic acid dye, a negative control tube, a positive control tube, a FTA membrane, quick drying liquid, and the like. The detection method has higher specificity, sensitivity and convenience than the TR-PCR detection method and has the advantages of low cost, no toxicity, high safety, convenient use, more accurate and quicker detection and high sensitivity and can replace the prior related detection methods, such as a pathological section method, an electron-microscopic observation method, an antibody detection method and a TR-PCR detection method. The on-site rapid high-sensitivity detection kit and the detection method can be both used in an indoor laboratory and a production site in the field, have significance for strengthening the TSV epidemiological monitoring and the disease control and prevention and have high popularization and application value.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Application of mycobacterium tuberculosis T cell epitope protein Rv1566c-444
PendingCN114671928AReduce false negativesHigh detection sensitivityAntibacterial agentsBacterial antigen ingredientsEpidemiological MonitoringImmunogenicity
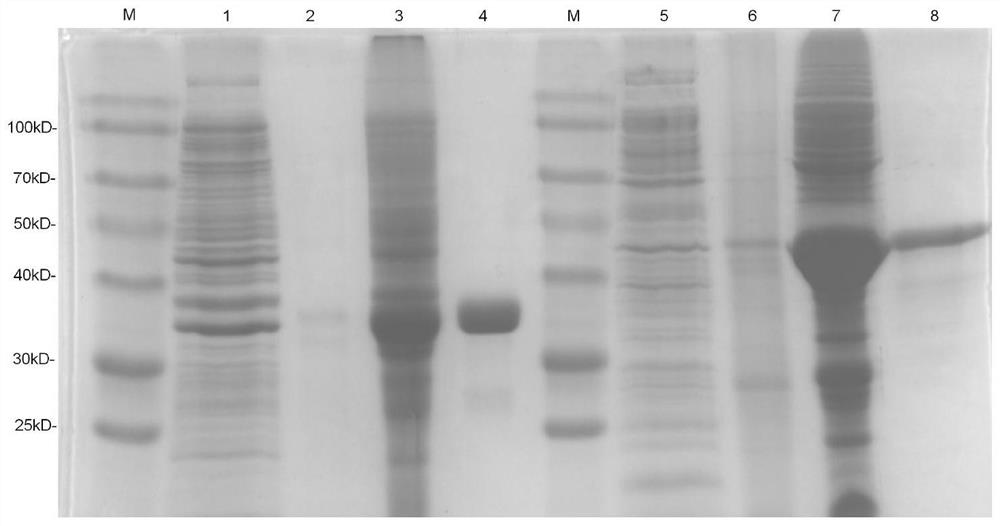
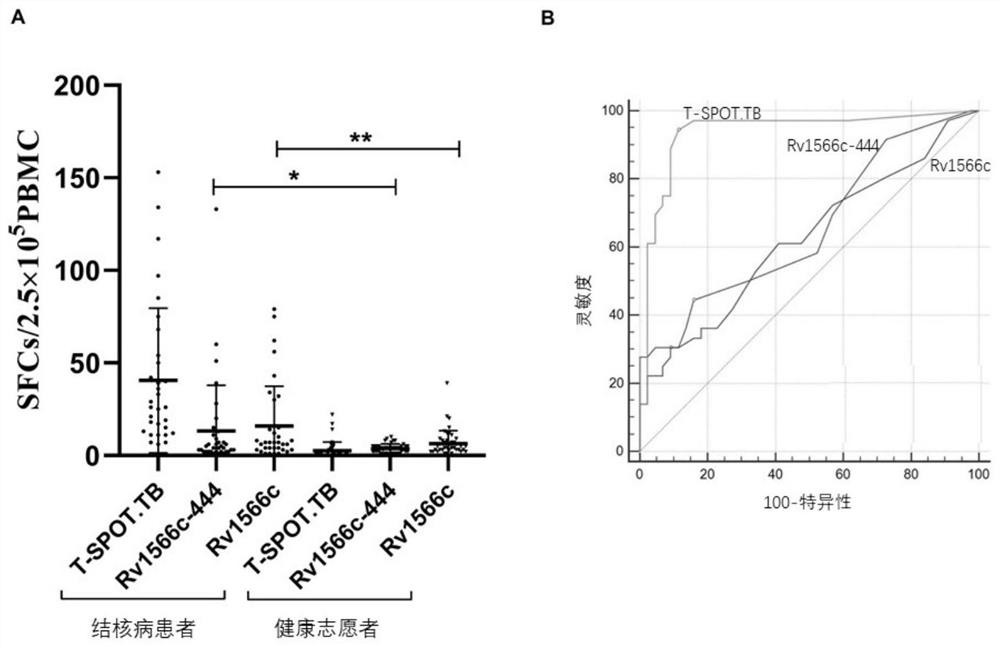
The invention relates to the technical field of molecular biology and immunology, and particularly discloses application of a T cell epitope protein Rv1566c-444 of mycobacterium tuberculosis. The invention provides an application of a mycobacterium tuberculosis T cell epitope protein Rv1566c-444 in preparation of a tuberculosis detection reagent and a vaccine. Wherein the amino acid sequence of the mycobacterium tuberculosis T cell epitope protein Rv1566c-444 is shown as SEQ ID NO.4, or is an amino acid sequence which is formed by replacing, deleting or adding one or more amino acids to the sequence and has the same antigenicity and immunogenicity. The mycobacterium tuberculosis T cell epitope protein Rv1566c-444 provided by the invention can reduce false negative caused by T cell stimulation by an intrinsic protein antigen of a kit, so that the detection sensitivity is improved. The cell immune response is good, and the vaccine can be widely applied to the related fields of auxiliary diagnosis of tuberculosis, epidemiological monitoring, infection screening and the like, and provides a new thought for the development of a new anti-tuberculosis vaccine.
Owner:ICDC CHINA CDC
Primer pairs, primer-probe compositions and their applications for identifying human adenovirus type 55
ActiveCN104073570BQuick checkReduce false positive resultsMicrobiological testing/measurementMicroorganism based processesEpidemiological MonitoringAgricultural science
The invention discloses a primer pair and a primer probe composition used for identifying human adenovirus type 55 and application thereof. A kit for aided identification and / or quantitative determination of human adenovirus type 55 comprises the specific primer pair composed of a single-stranded DNA molecule as shown in a sequence 1 in a sequence table and a single-stranded DNA molecule as shown in a sequence 2 in the sequence table. The kit further comprises a specific probe which has a nucleotide sequence as shown in a sequence 3 in the sequence table or shown in a reverse complementary sequence of the sequence 3 in the sequence table. According to the invention, the disadvantages of a great consumption amount of samples, complex experimental operation, low detection sensitivity, poor specificity and the like of traditional detection technology are overcome, and the kit has high application specificity, shows detection sensitivity as same as that of a 0.008PFU / reaction system and is applicable to high flux screening, especially to scientific research, clinical practice, epidemiological monitoring of viruses, etc.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com