Application of mycobacterium tuberculosis T cell epitope protein Rv1566c-444
A technology of rv1566c-444 and Mycobacterium tuberculosis, applied in the fields of molecular biology and immunology, can solve the problem of not being able to cause immune protection response, and achieve the effects of low cost, reducing false negatives, and improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 T cell epitope prediction and selection of T cell epitope-enriched sequences
[0041] The sequence of the Mycobacterium tuberculosis Rv1566c gene was obtained from the US National Center for Biotechnology Information (NCBI), see SEQ ID No. 1, and the corresponding protein sequence, see SEQ ID No. 2. T-cell epitope prediction software TEpredict and IEDB-AR were used to predict the Rv1566c gene that can bind to human HLA-A02 supertype alleles (including HLA-A *0201, *0202, *0203, *0206 motifs) T-cell epitopes, a total of 29 human T-cell epitopes were predicted in the Rv1566c gene, which can be divided into six epitope-concentrated regions. The present invention designs a sequence contained in the Rv1566c gene that retains all 29 T cell epitopes, and the targeting DNA sequence is named Rv1566c-444n, see SEQ ID No. 3, located at the 109-552 nucleotide position of the Rv1566c gene , the corresponding protein is defined as Rv1566c-444, see SEQ ID No.4. The distrib...
Embodiment 2
[0042] Example 2 Validation of T cell epitopes
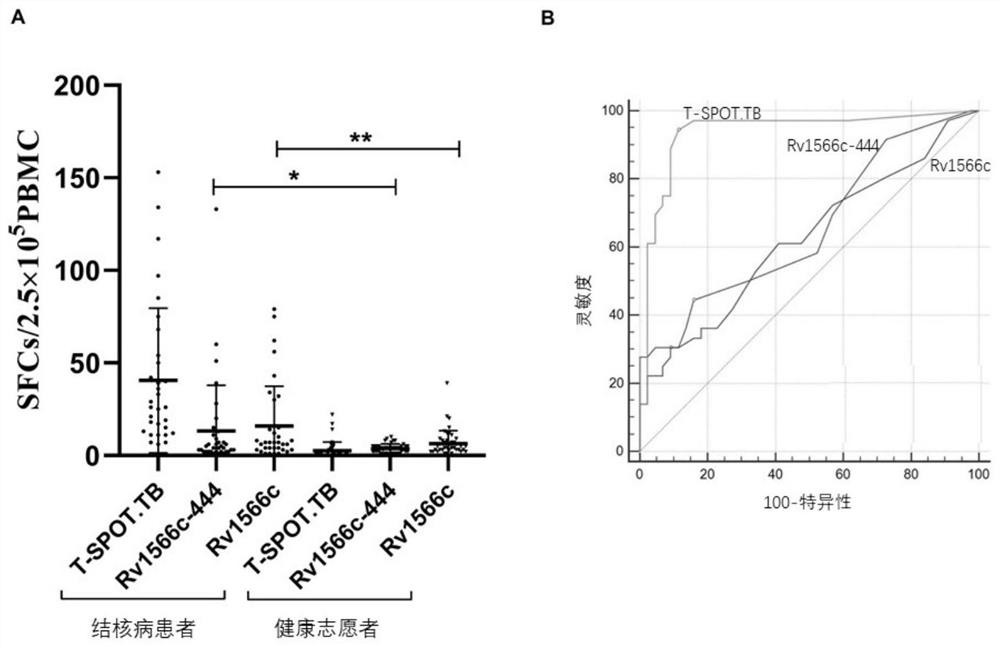
[0043] The gene sequences of the six epitope concentration regions of Rv1566c were sent to Shanghai Sangon Biotechnology Co., Ltd. to synthesize the corresponding six epitope peptides, and about 20 ml of peripheral blood from pulmonary tuberculosis patients were collected, and peripheral blood lymphocytes were separated. The peptides stimulated peripheral blood lymphocytes from 10 pulmonary tuberculosis patients respectively, and the ELISPOT test was carried out using the Mycobacterium tuberculosis effector T cell detection kit (enzyme-linked immunospot method) purchased from Beijing Jinhao Pharmaceutical according to the instructions. If the ELISPOT result of a pulmonary tuberculosis patient is positive according to the kit standard, this epitope polypeptide is determined to be a positive human T cell epitope polypeptide. The sixth polypeptide among the six epitope polypeptides of Rv1566c ( figure 1 D6) was identified as a pos...
Embodiment 3
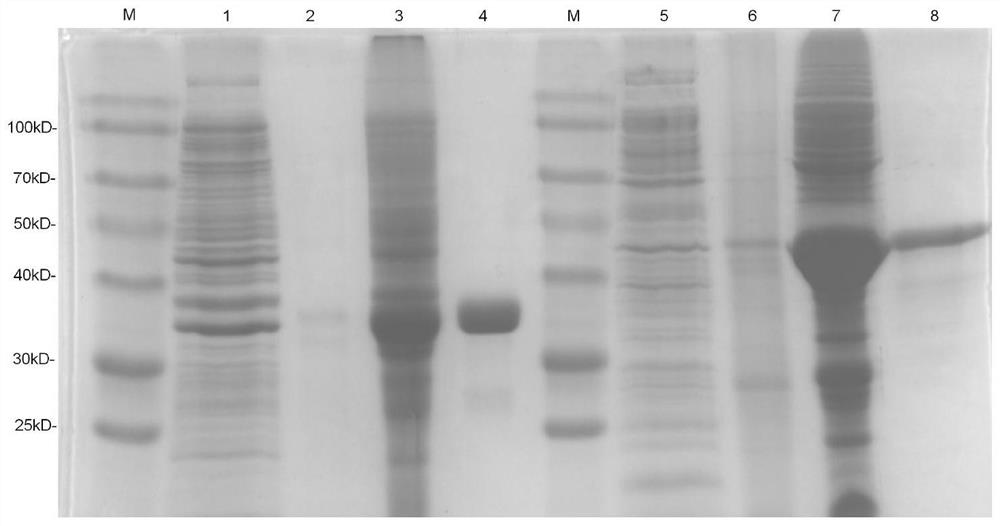
[0048] Example 3 Gene amplification, expression and purification of recombinant proteins pET-32a-Rv1566c and pET-32a-Rv1566c-444
[0049] Using the genomic DNA of Mycobacterium tuberculosis H37Rv as a template, the complete sequence of the Rv1566c gene and the epitope sequence Rv1566c-444n were amplified by PCR technology. After digestion with EcoRI and hind, it was ligated into pET-32a plasmid and transformed into Escherichia coli BL21(DE3). Protein expression was induced using 1 mM isopropyl β-d-thiogalactoside (IPTG) in the medium. After culturing at 37°C for 3.5 hours, the cells were collected by centrifugation at 4°C (4000 rpm, 10 min, 4°C). Expression levels and forms of Rv1566c and Rv1566c-444 in E. coli were detected by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by denaturation of the inclusion body proteins with 8M urea and nickel - Nitrilotriacetic acid (Ni-NTA) chromatography to purify the protein. Then, a low-concentration...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com