Application of indole-3-acetonitrile in preparation of medicine for treating or preventing influenza virus infection
A technology for influenza virus infection and influenza prevention, applied in the field of biomedicine, can solve problems such as unreported anti-influenza virus function, and achieve the effects of easy large-scale production, low cytotoxicity, and economical speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Effect of indole-3-acetonitrile on cell viability:
[0030] The effect of indole-3-acetonitrile on cell viability was determined by CCK-8 kit, and the optimal experimental concentration was screened out. details as follows:
[0031] The A549 cells in the 96-well plate grow to about 80%, incubate different concentrations (12.54 μM, 125.4 μM, 300 μM, 350 μM, 376.2 μM) of indole-3-acetonitrile, and cultivate them in a CO2 incubator at 37 ° C for 24 hours, and then protect them from light. Then add CCK-8 (10 μL / well). After culturing in a 37°C CO2 incubator for 2 hours, use a microplate reader to measure the OD 450nm absorbance at .
[0032] The results showed that the cell activity incubated with 376.2 μM indole-3-acetonitrile was 84.9%, and the cell activity incubated with 350 μM indole-3-acetonitrile was 98.8% (Table 1). Since there was no statistical difference between 98.8% and 100%, , 350 μM indole-3-acetonitrile was used for subsequent experimental studies.
[0...
Embodiment 2
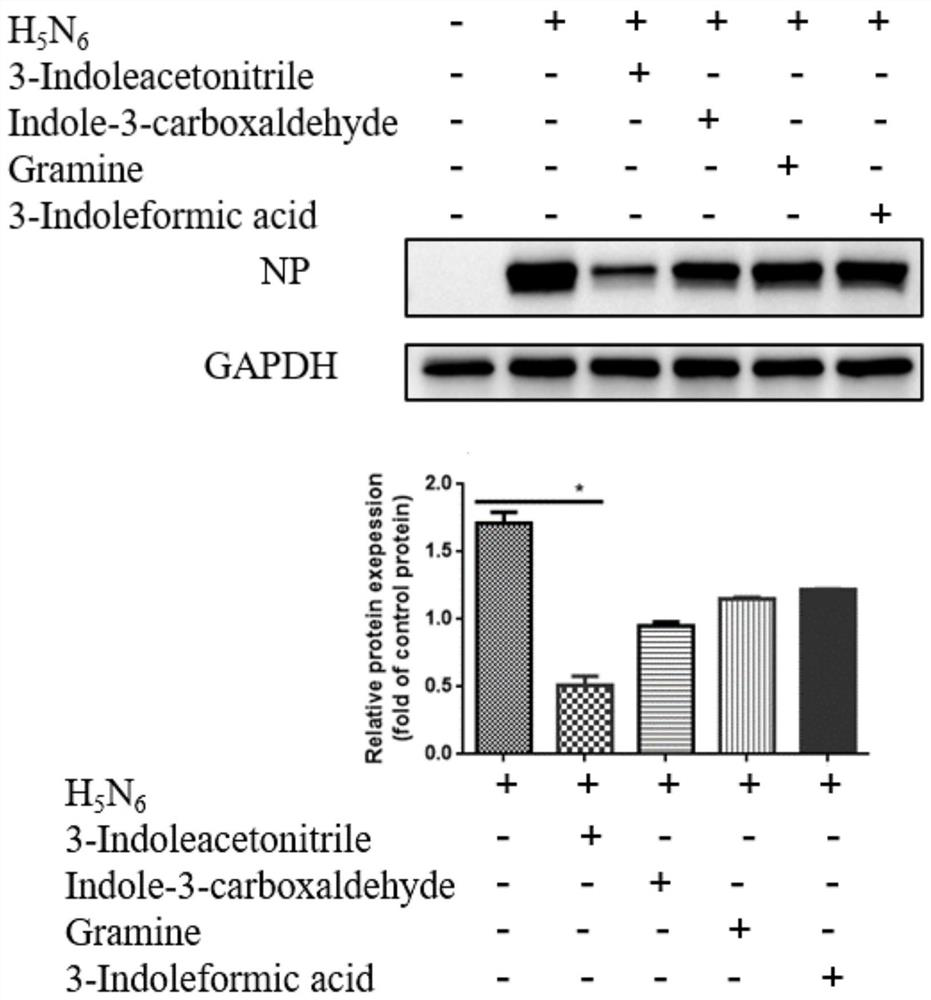
[0037] Preliminary screening of the inhibitory effect of indole-3-acetonitrile on the proliferation of influenza A virus in vitro:
[0038] 1. Fluorescence experiment.
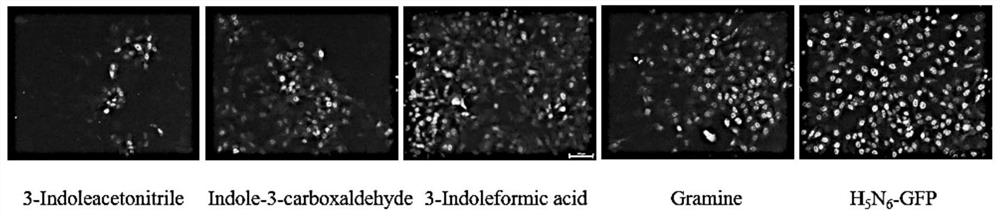
[0039] h 5 N 6 - After GFP virus infects cells, GFP fluorescence will increase with the proliferation of influenza virus. By observing the intensity of GFP fluorescence, it can indirectly reflect the proliferation of influenza virus. When the compound inhibits the proliferation of influenza virus, GFP fluorescence will be weakened. details as follows:
[0040] A549 in a 12-well plate was cultured in a CO2 incubator at 37°C until a cell monolayer was formed, and infected with H at 0.01MOI 5 N 6 -GFP, after 1h, they were replaced with different compounds (indole-3-acetonitrile, indole-3-carbaldehyde (Indole-3-carboxaldehyde), gramine (Gramine), indole-3-carboxylic acid (3-Indoleformic acid) acid)) cell maintenance solution (2% serum F-12), after 24 hours, the fluorescence was observed under an inverted micr...
Embodiment 3
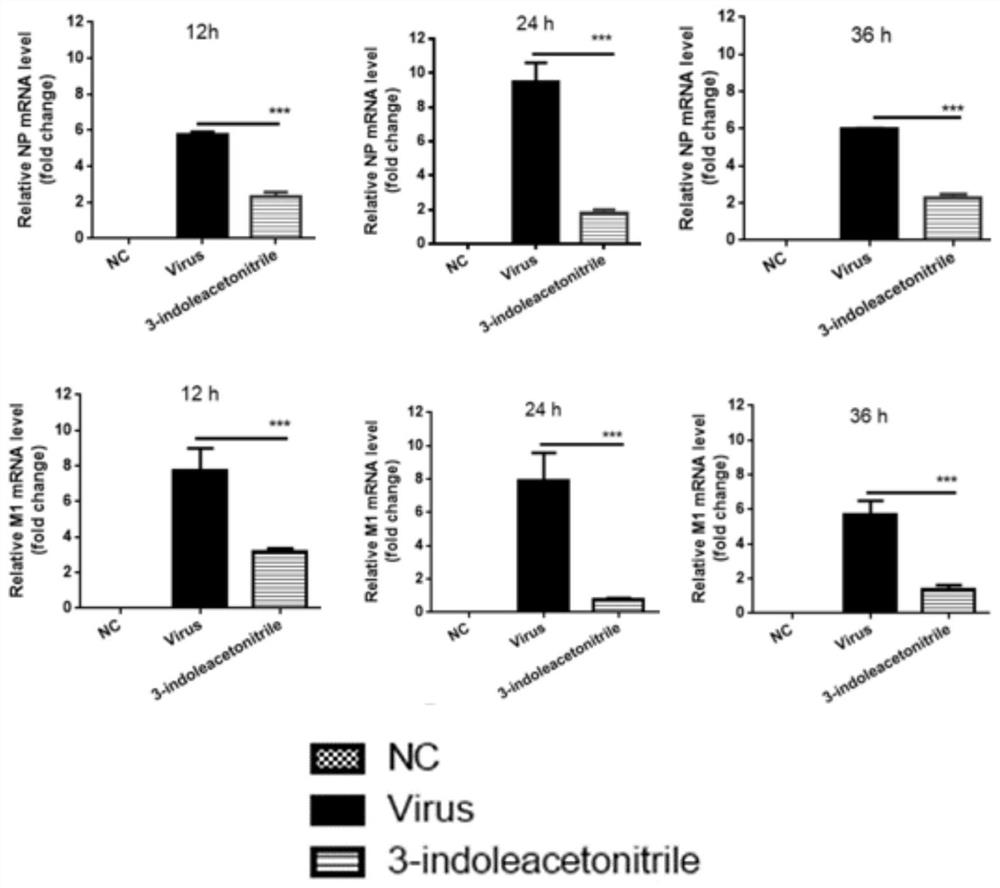
[0047] Effects of indole-3-acetonitrile on viral mRNA and host mRNA:
[0048] In order to study the effect of indole-3-acetonitrile on mRNA, a fluorescence quantitative test was carried out, as follows:
[0049] First, extract RNA and convert it into cDNA, use Trizol reagent to fully lyse the cells, then transfer to RNase-free EP tubes, fully invert, and ice-bath for 10 minutes. Add 200 μL of chloroform to each tube, vortex and centrifuge. Take the supernatant to a new RNase-free EP tube, add an equal volume (500 μL) of isopropanol, mix gently, let stand and centrifuge, and discard the supernatant. Add 900 μL of ethanol, vortex and centrifuge, discard the supernatant. Blow dry while working on the clean bench. Add the DNA digestion system, bathe in a water bath at 37°C for 1 hour, then add 5 μL of DNAStop Solution to each tube, flick it briefly, and then bathe in water at 65°C for 15 minutes. Take a small amount to measure the RNA concentration. After the preparation of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com