RT-LAMP (reverse transcription loop-mediated isothermal amplification) detection primers for specifically detecting SIV (swine influenza virus), application of primers, detection reagent and method
A 1. RT-LAMP, swine flu virus technology, applied in biochemical equipment and methods, DNA/RNA fragments, recombinant DNA technology, etc., can solve the problem of weak positive judgments that are not accurate enough, difficult to detect in broad spectrum, and easy to pollute the environment and other problems, to achieve the effect of real-time observation of results, high detection sensitivity, and avoidance of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1, the screening of primer combination
[0102] 1. Design LAMP primers according to the specific segment of the NP gene sequence of the swine influenza virus (MG983192.1) in GenBank, and select the optimal primer combination as follows:
[0103] The primer set SIV1 sequence is as follows:
[0104] Outer primer SIV1-F3: 5'-TCTGGAGGGGTGAAAATGGA-3'
[0105] Outer primer SIV1-B3: 5'-GTGCAACTGATCCCCTCAG-3'
[0106] Internal primer SIV1-FIP:
[0107] 5'-GCCCTCTGGGCAGCTGTTTGCGAAGGACAAGGGTTGCTTA-3'
[0108] Internal primer SIV1-BIP:
[0109] 5'-AAGTCGAAACCCAGGAAACGCTAATGAGTGCTGACCGTGC-3'
[0110] According to the specific segment of the NP gene sequence of swine influenza virus (MG836780.1) in GenBank, LAMP primers were designed, and the optimal primer combination was selected as follows:
[0111] The primer set SIV2 sequence is as follows:
[0112] Outer primer SIV2-F3: 5'-AGAAAGTGATTCCAAGAGGA-3'
[0113] Outer primer SIV2-B3: 5'-GGTTGCTCTTTTCAAAAGGGA-3'
...
Embodiment 2
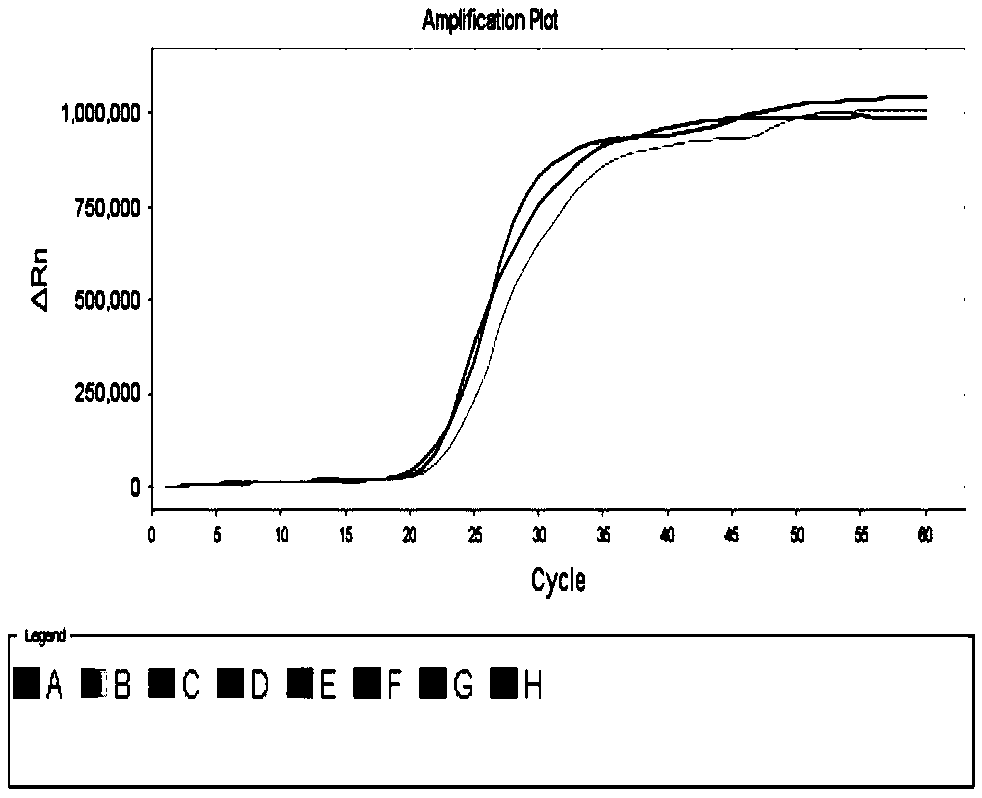
[0148] Embodiment 2, specificity experiment
[0149] Swine influenza virus (SIV), swine fever virus (CSFV), porcine pseudorabies virus (PRV), porcine blue ear virus American type highly pathogenic strain (HP-PRRSV), porcine blue ear virus American type classic strain (PRRSV), Each test sample of porcine parvovirus (PPV1) and porcine circovirus (PCV2) carries out the following steps respectively:
[0150] 1. Extract the genomic DNA or RNA of the sample to be tested.
[0151] 2. Using the viral genome extracted in step 1 as a template, the primer set screened in Example 1 was used to perform reverse transcription-loop-mediated isothermal amplification.
[0152] Reaction system (20 μL): 10 μL reaction solution, 2.96 μL primer mixture, 2 μL genomic DNA / RNA (5pg-50pg), 0.5 μL AMV reverse transcriptase (added in the RNA template system), filled to 20 μL with RNase-free water. The primer mixture is the mixture of each primer in the primer set. In the reaction system, 0.12 μL each ...
Embodiment 3
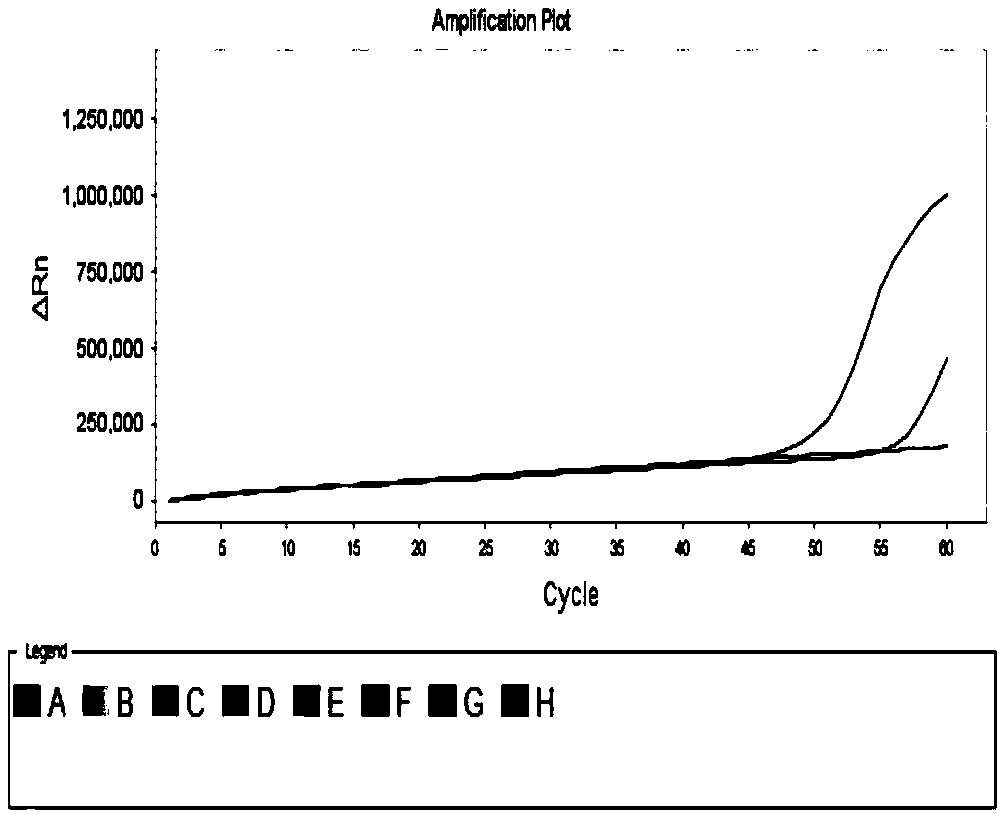
[0157] Embodiment 3, sensitivity
[0158] Sample to be tested: the plasmid DNA of swine influenza virus prepared in Example 1.
[0159] 1. Extract the plasmid DNA of the sample to be tested, and perform gradient dilution with sterilized pure water to obtain each dilution.
[0160] 2. Using the dilution obtained in step 1 as a template, the primer combination prepared in Example 1 was used to perform loop-mediated isothermal amplification.
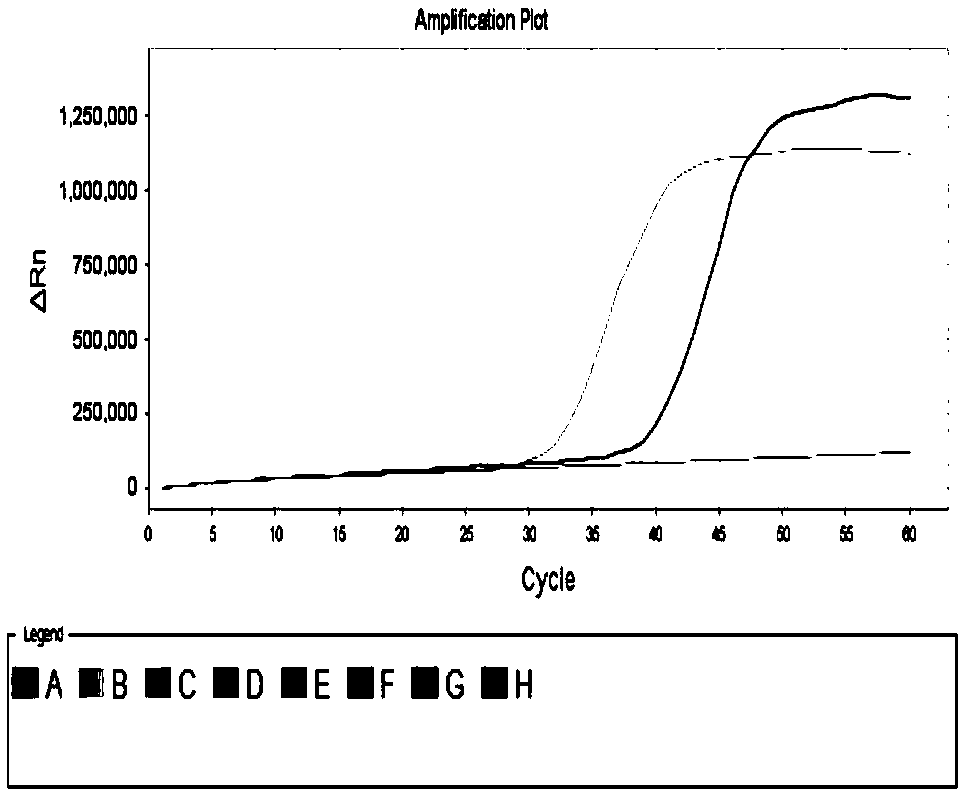
[0161] Reaction system (20 μL): 10 μL reaction solution (product of Boao Biological Group Co., Ltd., catalog number: CP.440020), 2.96 μL primer mixture, 2 μL diluent (the copy number of plasmid DNA contained in 1 μL diluent is 5× 10 2 、10 2 or 10 1 ), replenish water to 20 μL. The primer mixture is the mixture of each primer in the primer combination. In the reaction system, 0.12 μL each of 0.3 mM outer primers F3 and B3; 0.96 μL each of 2.4 mM inner primers FIP and BIP; 0.4 μL each of 1 mM loop primers LF and LB, if the primer lacks ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com