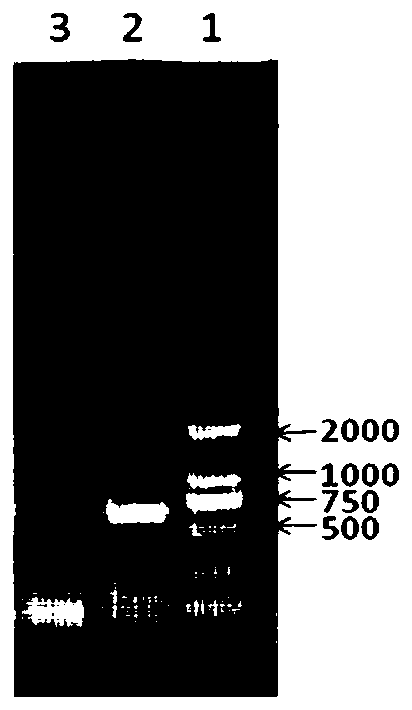
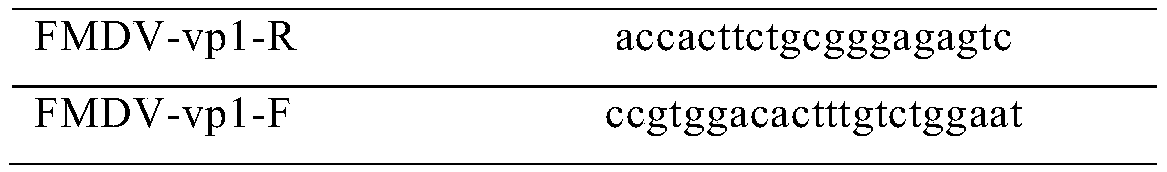
Triple inactivated vaccine for pigs and preparation method and application thereof
An inactivated vaccine and inactivated technology, applied in veterinary vaccines, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of strong infectivity, affecting the growth performance of pigs, and high morbidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
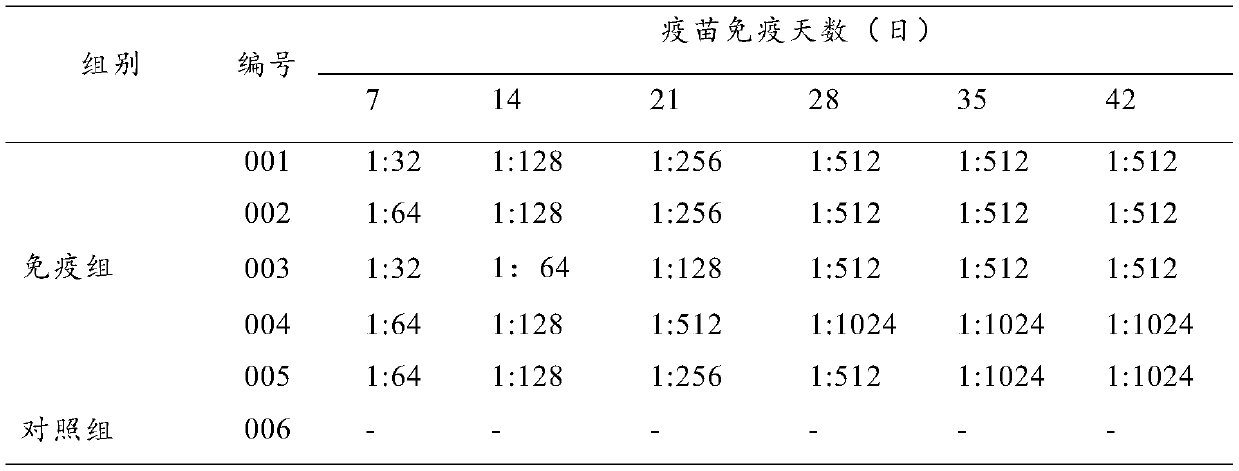
[0024] Embodiment 1 pig uses the preparation of triple inactivated vaccine
[0025] 1 poison seed
[0026] Porcine Seneca virus, its microbial preservation number is: CGMCC No.18851; classification name: Seneca virus; preservation unit: General Microbiology Center of China Committee for the Collection of Microorganisms; preservation time is October 29, 2019; preservation Address: No. 3, Yard No. 1, Beichen West Road, Chaoyang District, Beijing.
[0027] H3N2 subtype swine influenza virus, its microbial preservation number is: CGMCC No.14740; classification name is: H3N2 subtype swine influenza virus; preservation unit: General Microbiology Center of China Committee for the Collection of Microbial Cultures; preservation time is November 2017 16th; Preservation address: No. 3, Yard No. 1, Beichen West Road, Chaoyang District, Beijing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com