Method for testing efficacy of porcine Seneca virus disease inactivated vaccine by using rabbits
A technology of inactivated vaccines and viral diseases, applied in veterinary vaccines, vaccines, viruses, etc., can solve the problems of no longer safe and reliable, rising prices, and difficulties in purchasing experimental pigs, and achieve simple operation and low feeding costs , the effect of low purchase cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the preparation of porcine Seneca virus inactivated vaccine
[0025] This embodiment chooses to use SVV / CH / ZZ / 2016CGMCC No.14886 (CN110551694A) as the strain to prepare Seneca virus liquid, obtain the vaccine in the following examples after inactivation and emulsification, specifically comprising the following steps:
[0026] Inoculate the Seneca virus strain at a cell density of 3.0 × 10 6 , PK15-JY suspension cells with cell viability above 95% (porcine kidney suspension cells, provided by Jinyu Baoling Biopharmaceutical Co., Ltd.), placed in 5% CO at 37°C 2 After culturing in the incubator for 3 days, the virus culture medium was harvested by repeated freezing and thawing three times, and BEI (diethyleneimine, Sigma) was added to make the final concentration 0.003mol / L, and inactivated at 26°C for 48 hours. According to the content of 4% (V / V), 50% sodium thiosulfate solution was added to block the inactivation, and the inactivated antigen was obtained...
Embodiment 2
[0027] Embodiment 2, the preparation of Seneca virus liquid
[0028] This embodiment selects and uses SVV / CH / ZZ / 2016 CGMCC No.14886 (CN110551694A) to prepare Seneca virus liquid as virus strain, is used for the challenge poison in following embodiment, specifically comprises the following steps:
[0029] The Seneca virus strain was inoculated on PK-15 cells (porcine kidney cells, provided by Jinyu Baoling Biopharmaceutical Co., Ltd.) overgrown with a single layer, and placed in 5% CO at 37°C. 2 Cultivate in the incubator for 5 days, freeze and thaw repeatedly 3 times to harvest the virus culture medium, record it as the F1 generation, and store it at -20°C. Take the F1 generation of the harvested virus culture medium, inoculate PK-15 cells that have grown into a monolayer according to the content of 10% (V / V), and place them in 5% CO at 37°C. 2 Cultivate in an incubator, observe cytopathic changes (CPE) every day, harvest the virus culture liquid 48 hours after inoculation, a...
Embodiment 3
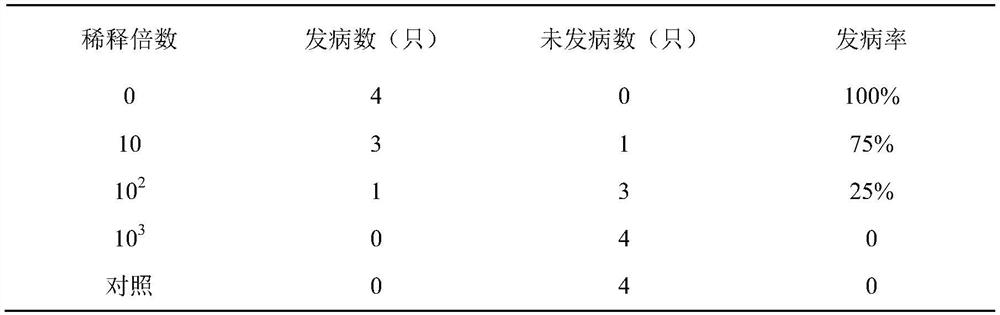
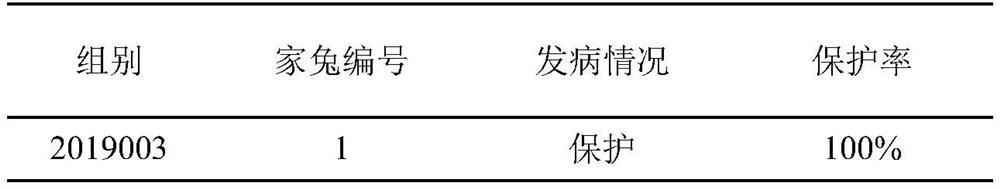
[0030] Embodiment 3, determination of rabbit challenge dose
[0031] This embodiment utilizes the Seneca virus liquid that above-mentioned embodiment 2 obtains to carry out challenge test to rabbit, to determine the challenge dose of rabbit, specifically comprises the following steps:
[0032] 3.1. Selection of experimental animals: 20 healthy and susceptible rabbits of 1.0-2.0 kg were selected, and the serum neutralizing antibody of Seneca virus was detected <1:2, purchased from Eternal Agriculture and Animal Husbandry Development Co., Ltd., Dalat Banner, Ordos City;
[0033] 3.2. Preparation of poison for attacking poison: the poison price prepared in Example 2 is 10 7.5 TCID 50 / 0.1ml of the virus liquid is the virus stock solution for gradient dilution to obtain a 10-fold dilution, 10 2 double dilution and 10 3 Two-fold dilutions are used to challenge the rabbits respectively;
[0034] 3.3. Virus challenge: 20 healthy susceptible rabbits were randomly divided into 5 gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com