Duplex real-time fluorescent quantitative PCR detection kit for Seneca virus A and foot and mouth disease virus
A real-time fluorescent quantitative, foot-and-mouth disease virus technology, applied in the direction of microbial determination/inspection, recombinant DNA technology, biochemical equipment and methods, etc., can solve the difficulty of clinical diagnosis, inability to distinguish SVA infection and FMDV infection and other problems, to ensure safety The effect of high reliability and rationality, easy operation and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Design of primers and TaqMan probes for detection of Seneca virus (SVA) and foot-and-mouth disease virus (FMDV) using real-time fluorescent quantitative PCR technology
[0048] Retrieved from NCBI's Nucleic Acid Database GenBank (http: / / www.ncbi.nlm.nih.gov) to obtain the complete genome sequence of Seneca virus (GeneBank serial numbers: NC_011349, KT321458, KX173339, KX173338, KX173340, KX751943, KX751944, KY747510 , KY038016, KY747511, KY747512, KX751945, KX751946, KX377924, KY419132, DQ641257, KU051392, KT757280, KU051391, KY486158, KY486165, KC667560, KR063109, KR063107, KY368743) and the sequence of the foot-and-mouth disease virus full genome (AF50 GeneBank, 6822.2) KX712091.1, HQ412603.1, AJ539141.1, AY390432.1, AY304994.1, GQ406249.1, KT968663.1, HQ632773.1), after comparison with DNA Star software, according to the design principles of primers and TaqMan probes , Respectively select the relatively conservative nucleotide sequence between different strai...
Embodiment 2
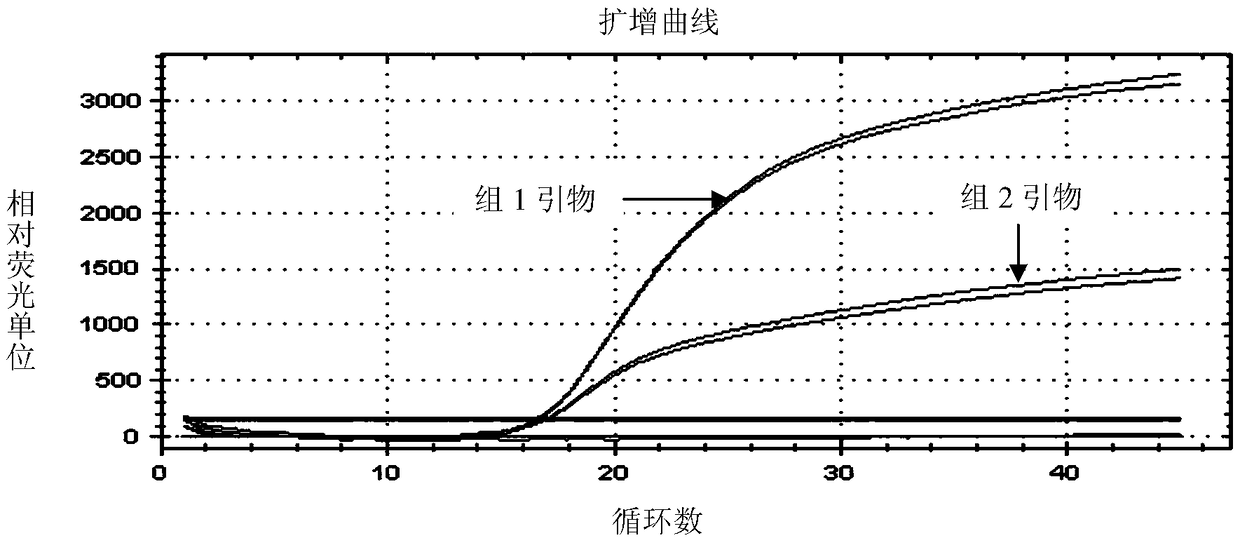
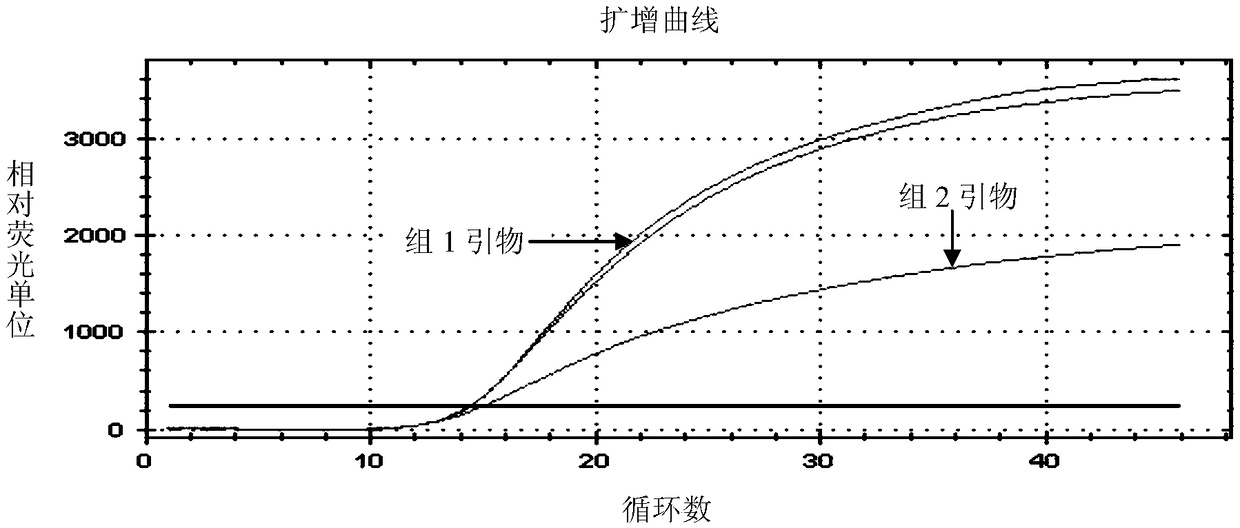
[0073] Example 2. Double real-time fluorescent quantitative PCR detection of Seneca virus and foot-and-mouth disease virus using primers of group 1 and TaqMan probe
[0074] 1. Extract the genomic RNA of Seneca virus and foot-and-mouth disease virus
[0075] Seneca virus cell culture (the cell-passage virus isolated and identified by our laboratory, used to obtain standard and positive control), foot-and-mouth disease virus cell culture (Jinyu Baoling company MYA98 vaccine strain, used to obtain standard And positive control) and the sample to be tested are used as the sample to be extracted, and the genomic RNA of the sample to be extracted is extracted. The specific extraction method refers to the AXYGEN kit (Axyprep TM The introduction of Body Fluid Viral DNA / RNA Miniprep Kit, AXYGEN Company) includes the following steps:
[0076] (1) According to the kit instructions, prepare isopropanol containing 1% glacial acetic acid in advance and add the specified concentration of absolut...
Embodiment 3
[0122] Example 3. Preparation of a dual real-time fluorescent quantitative PCR detection kit for Seneca virus and foot-and-mouth disease virus
[0123] Based on Example 1 and Example 2, the dual real-time fluorescent quantitative PCR detection kit for Seneca virus and foot-and-mouth disease virus of the present invention includes primers (SVA-F) for dual real-time fluorescent quantitative PCR detection of Seneca virus and foot-and-mouth disease virus. , SVA-R, FMDV-F and FMDV-R) and TaqMan probes (SVA-P and FMDV-P).
[0124] The 25μL dual real-time PCR detection system when using the kit is: real-time fluorescence quantitative one-step PCR reaction solution 2×One Step RT-PCR Buffer III 12.5μL, TaKaRa Ex Taq HS 0.5μL, PrimeScript RT Enzyme Mix II 0.5μL , SVA-F (10μM) 1.0μL, SVA-R (10μM) 1.0μL, SVA-P (10μM) 1μL, FMDV-F (10μM) 1.0μL, FMDV-R (10μM) 1.0μL, FMDV-P (10μM) ) 1.5μL, template RNA 2.0μL, RNA-free H 2 O 3.0μL.
[0125] To facilitate detection, the kit can also include a positi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com