Meningococcus antigen composition and applications thereof
A meningitis and antigen technology, applied in the field of vaccines, can solve the problems of vaccines with increased potency and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1 Construction of recombinant expression engineering bacteria
[0096] The genomes were extracted from the cultures of three clinical isolates of meningococcus, and the first fHbp (named fHbp-1, namely variant 1) and the second fHbp ( Named fHbp-2, which is variant 2) and NHBA gene. The PCR amplification forward primer and reverse primer are listed in the table below. The forward primer and reverse primer introduce the restriction enzyme sites of Nde I and BamH I (fHbp) or Nhe I and BamH I (NHBA), respectively. The underlined display. Recombinant fHbp and NHBA proteins were expressed in E. coli in the form of His-tag fusion and plasmid pET-28a (Novagen) was used as the expression vector.
[0097]
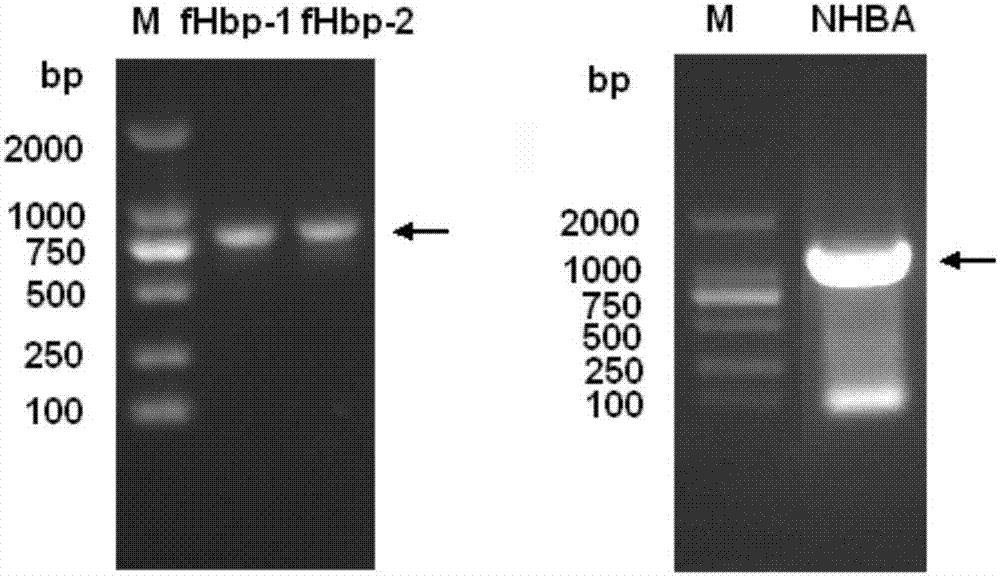
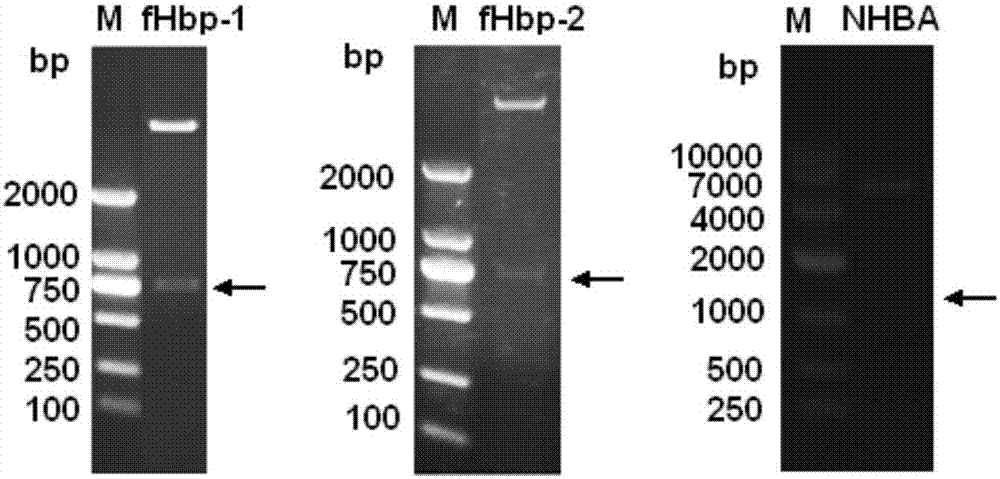
[0098] Cloning of fHbp-1 and fHbp-2: PCR was performed from bacterial genomic DNA with rTaq DNA polymerase (TaKaRa). The PCR reaction conditions were: 95°C for 5 min; 95°C for 1 min, 60°C for 1 min, 72°C for 1 min, 35 cycles; 72°C for 8 min extension. The amplified pro...
Embodiment 2
[0102] Example 2 Fermentation culture
[0103] Fermentation culture of recombinant fHbp-1 and fHbp-2. Transform the recombinant plasmid into BL21(DE3) competent, pick the monoclonal colony on the plate, inoculate it into 5ml LB liquid medium containing kanamycin, culture with shaking at 37℃ overnight, and transfer 250ml containing kanamycin the next day In an Erlenmeyer flask of LB liquid medium, culture with shaking at 37℃ to 600nm absorbance value A 600 The value is 0.6, add IPTG to a final concentration of 0.6mmol / L, continue shaking culture for 5 hours, centrifuge at 6000rpm for 10 minutes, and collect the bacteria.
[0104] Fermentation culture of recombinant NHBA. Transform the recombinant plasmid into BL21(DE3) competent, pick the monoclonal colony on the plate, inoculate it into 5ml LB liquid medium containing kanamycin, culture with shaking at 37℃ overnight, and transfer 250ml containing kanamycin the next day In an Erlenmeyer flask of LB liquid medium, culture with shak...
Embodiment 3
[0105] Example 3 Purification of target protein
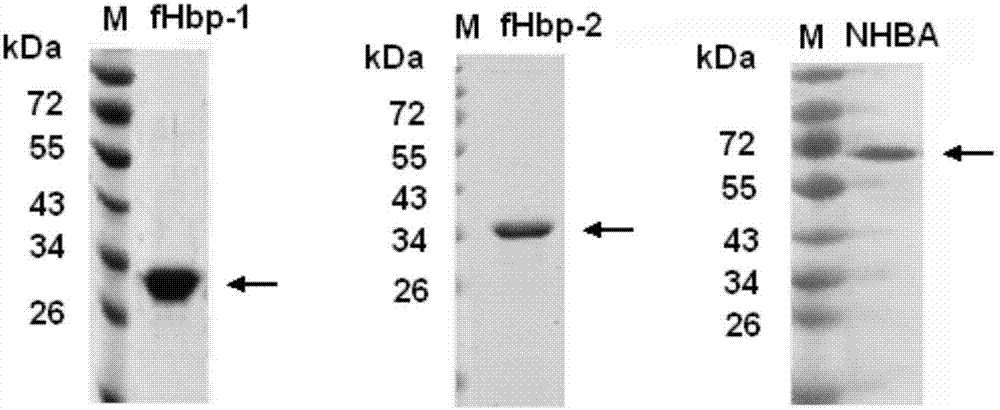
[0106] Resuspend the bacterial body fluid with a nickel column equilibration buffer and place it in a mixture of ice and water, and ultrasonically break the bacteria. After sterilization, the supernatant was harvested by centrifugation. Ni 2+ The crude product was purified by affinity chromatography column, the recombinant fHbp-1 and fHbp-2 were further purified by gel filtration chromatography, and the recombinant NHBA was further purified by CM ion column. See the SDS-PAGE electrophoresis pattern of the purified recombinant fHbp-1, fHbp-2 and NHBA protein Figure 4 . The purified protein is stored at -20°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com