Subunit vaccine for porcine reproductive and respiratory syndrome as well as preparation method and application of subunit vaccine
A subunit vaccine and porcine PRRS technology, which is applied in the field of porcine PRRS subunit vaccine and its preparation, can solve problems such as irritation, and achieve the effects of improving immunogenicity, good protection effect, and easier separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Construction of target protein expression vector
[0041] 1. Obtain the target protein sequence:
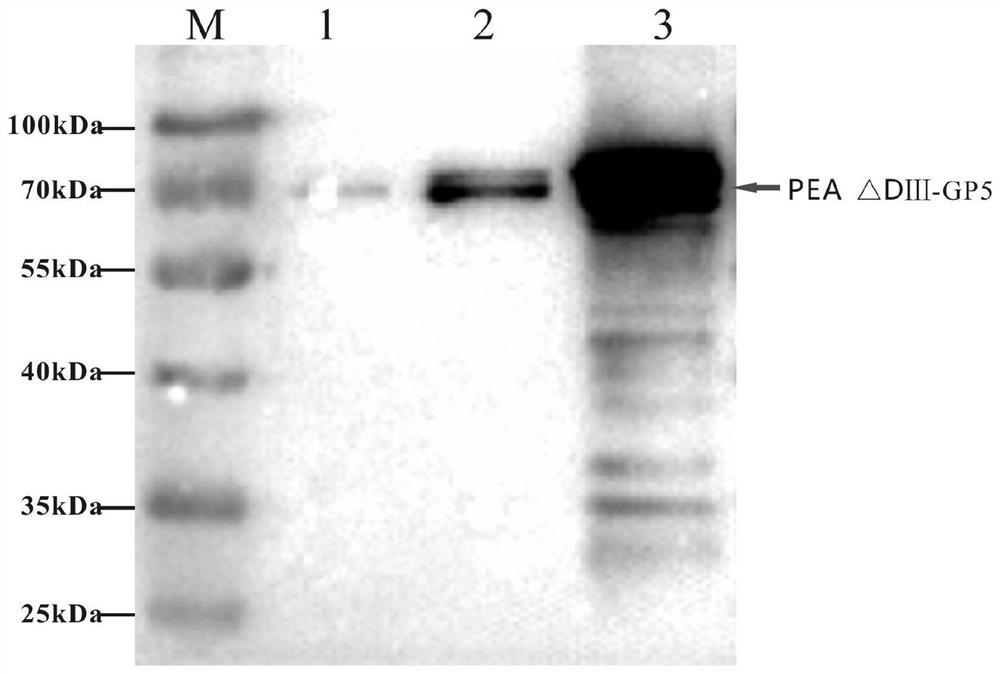
[0042]Get the gene sequence of PRRSV NADC30-like strain from NCBI (Accession: MH651743), select the nucleotide sequence of GP5 protein and optimize it; get the gene sequence of Pseudomonas aeruginosa from NCBI (GenBank: CP039293), select the exotoxin The translocation domain of PEA (remove its DomainⅢ, hereinafter referred to as PEA△DⅢ) sequence; connect PEA△DⅢ and the optimized GP5 gene sequence in series, add 8×His tag sequence, add NdeI and C-terminal respectively XhoI restriction site, gene sequence of biosynthetic recombinant protein (PEA△DⅢ-GP5) (as shown in SEQ ID No.1).
[0043] 2. Link the synthetic fusion protein pUC-PEA△DⅢ-GP5 sequence into the expression vector pFastBacdual with NdeI and XhoI restriction sites:
[0044] (1) Enzyme digestion of pUC-PEA △DⅢ-GP5 and insect baculovirus expression vector pFastBacdual (purchased from Fenghui Biotechnology, produ...
experiment example 1
[0066] Experimental Example 1 Safety Test of PEA△DⅢ-GP5 Subunit Vaccine on Mice
[0067] Ten Balb / C mice aged 4-5 weeks were purchased and divided into 2 groups, 5 mice in each group, which were the immune group and the control group, respectively. The immunized group was inoculated with 200 μL of the PEA△DⅢ-GP5 subunit vaccine prepared in Example 1 by calf intramuscular injection on day 0 and day 14 respectively; the blank control group was injected with the same volume of vaccine control in the same way. Continuous observation for 14 days after immunization showed that the test mice had normal spirit and appetite, and were in good health without any adverse reaction, which indicated that the subunit vaccine was safe for mice. The results of the safety test on mice are shown in Table 1.
[0068] Table 1
[0069]
experiment example 2
[0070] Experimental example 2 PEA△DⅢ-GP5 subunit vaccine safety and effectiveness test on piglets
[0071] 1. Safety and effectiveness test of PEA△DⅢ-GP5 subunit vaccine on piglets:
[0072] Ten 45-day-old PRRSV-negative piglets were selected and divided into two groups, 5 in the immunization group and 5 in the control group. The immunized group was intramuscularly injected with 1.0 ml of the PEA△DⅢ-GP5 subunit vaccine prepared in Example 1 behind the ears on the 0th day and the 14th day, and the blank control group was not immunized. After 14 days of continuous observation, the piglets did not show any clinical symptoms, and their spirit, appetite, and body temperature were all normal, without any adverse reactions. The results showed that the subunit vaccine was safe for piglets.
[0073] The specific results of the piglet safety test are shown in Table 2.
[0074] Table 2
[0075]
[0076] 2. Immune efficacy test of PEA △DⅢ-GP5 subunit vaccine
[0077] Ten 45-day-old...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com