A kind of porcine epidemic diarrhea recombinant baculovirus genetically engineered subunit vaccine and its preparation method and application
A technology for recombinant baculovirus and porcine epidemic diarrhea, applied in genetic engineering, botany equipment and methods, microbial-based methods, etc., can solve the problems of low effective antigen content, low expression level, poor immunogenicity, etc. , to achieve good immunogenicity, high expression level and high expression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
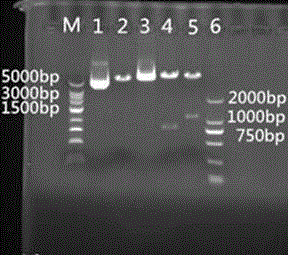
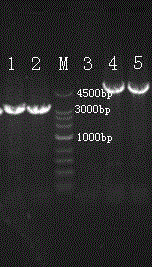
Image
Examples
Embodiment 1
[0030] Construction of recombinant vectors Bacmid-S1p-M and Bacmid-S1
[0031] 1. Acquisition of PEDVS1 gene, S1 partial antigen gene and M gene: Design a pair of specific primers according to the gene sequence of PEDV newly isolated strain, the primer sequence is as follows:
[0032] S1-P1: 5'CGGAATTCCAAGATGTCACTAGGTGCCAGTCTA3'
[0033] S1-P2: 5'CCCTCGAGCTATCTAATACTCATACTAAAGTTGGTGGG3'
[0034] M-P1: 5'GAAGGCCTATGCATCACCATCACCATCACGATTA3'
[0035] M-P2: 5'CCAAGCTT TTA GACTAAATGAAGCACTTTCTC3'
[0036] S1p-P1:5'CCCTCGAG ATG GTACTTCTAACACTTAGCCTACCAC3'
[0037] S1p-P2:5'CATGCATGC CTAs AGGATCTGAGGAATTACTGCAAACA3'
[0038] The total RNA was extracted from the positive PEDV disease material, and each downstream primer P2 was used as the specific primer for RT; then the cDNA product was used as the template to amplify the whole S1 gene, S1p (partial epitope of S1 gene) by PCR technology. ) and M gene; wherein the nucleotide sequence of the coding gene S1p of the partial S...
Embodiment 2
[0043] Obtaining of Recombinant Baculovirus Bacmid-S1p-M and Bacmid-S1
[0044] 1.1 Recovery and subculture of Sf9 insect cells
[0045] 2. Transfect Sf9 cells with recombinant Bacmid-S1p-M and Bacmid-S1 plasmids by 2×10 5 Inoculate Sf9 cells on a 12-well cell culture plate at a density of 12, and transfect when the cells grow to 80-90% full, discard the old cell culture medium, and replace it with serum-free Grace cell culture medium; in sterile 1.5 Prepare the following solutions in a mL centrifuge tube: Solution A: Dilute 1.6 μg DNA to 100 μL with GRACE Incomplete Cell Culture Medium; Solution B: Dilute 4.0 μL Lipofectanmine 2000 to 100 μL with GRACE Incomplete Cell Culture Medium; Mix Solution A and B. Stand at room temperature for 20 minutes to form a complex of liposome and DNA; slowly add the complex of liposome and DNA to the cell surface drop by drop (do not discard the nutrient solution); after 4-6 hours, put the transfection solution into the The cell culture medi...
Embodiment 3
[0046] The preparation of embodiment 3 vaccines:
[0047] After the recombinant baculovirus Bacmid-S1p-M and recombinant baculovirus Bacmid-S1 were obtained through Example 2, the M protein and the S1 protein were expressed using a virus expression system. The step is to expand the culture of recombinant baculovirus Bv-S1p-M or recombinant baculovirus Bv-S1, purify and recover to obtain S1 protein or S1p protein and M protein; expand the culture to obtain recombinant baculovirus Bv-S1 with a suitable titer After the virus, when the cells are in the mid-log phase (the density is 1-2×10 6 Cells / mL) were inoculated with infected cells, and the insect cells were cultivated under serum-free conditions, which would facilitate the purification of the target protein in the later stage. However, generally according to needs, 0.1-0.5% FBS or BSA should be added at the late stage of infection to protect the recombinant protein from hydrolysis. After the cells were inoculated with the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com