Lipid cochleate carrier based on aluminum ions
A technology of lipid volume and aluminum ions, which is applied in the field of biomedicine, can solve the problems of redness and swelling at the inoculation site, and achieve the effect of strong adjuvant function, enhanced stability and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
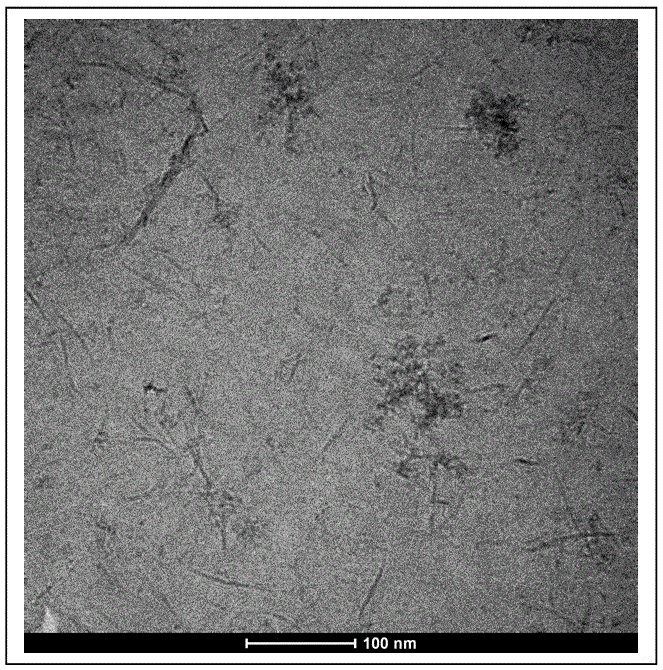
[0030] The preparation method of novel lipid roll carrier is to adopt negatively charged phospholipid as membrane material, prepare liposome by various methods; add aluminum ion (Al 3+ ), causing the liposome phospholipid bilayer to sweep to form a helical lipid volume ACO, which is the liposome fusion method. Different phospholipids are used as membrane materials, or different preparation processes are used, resulting in different ACO particle sizes.
[0031] To enhance vaccine efficacy, ACO is further encapsulated or combined with other immune adjuvants, such as lipid A (lipid A), monophospholipid A, CpG-ODN, saponin, squalene, imiquimod, etc.; In order to improve the efficiency of vaccine delivery, the surface of ACO is modified with the corresponding ligand of APC receptor, for example, ACO is modified with mannose group.
[0032] ACO products are in the form of carrier aqueous solution; in order to improve stability, anhydrous products are further prepared by spray dryin...
Embodiment 1
[0035] Preparation of Encapsulated Antigen ACO Vaccine by Liposome Fusion Method
[0036]Use OVA (model antigen) solution as the water phase, SPS / MPLA (50:1, mole ratio, SPS concentration 1% (g / mL)) as the membrane material, SPS / OVA (20:1, mass ratio), and Thin-film dispersion-extrusion method (passing through 100 nm porous membrane) to prepare 100 nm liposomes; add 20 mM AlCl 3 solution, the liposomes fuse to form a new type of lipid volume ACO. Microscopic observation shows that ACO is a rod-shaped particle. The average particle size detected by DLS is 960 nm, the zeta potential is 20 mV, and the encapsulation efficiency is about 95%. In vitro experiments showed that mouse myeloid dendritic cells (BMDCs) efficiently internalized fluorescently labeled ACO. Mice were inoculated intramuscularly with OVA-ACO (inoculation dose 5 µg), compared with the control group (Ca 2+ Compared with traditional lipid volume group and aluminum salt adjuvant group), higher levels of INF-γ, OV...
Embodiment 2
[0039] Mannose-modified ACO vaccine prepared by emulsification and freeze-drying
[0040] Using 2.5 mL of 5% sucrose as the water phase and 0.5 mL of SPC cyclohexane (cyclohexane) as the oil phase, the water phase and the oil phase (5:1, v / v) were mixed and ultrasonically emulsified to prepare microemulsions; then frozen Dry, remove solvent; freeze-dried product with AlCl 3 Solution hydration (SPS / Al 3+ = 1:5, mol / mol), that is, the formation of ACO; followed by OVA, mannose-PEG2000-DSPE (MPE) (SPS / OVA = 20:1, mass ratio, SPS / MPE = 50:1, mole ratio,) Mix and incubate at 40 °C for 0.5h to form mannose-modified lipid rolls (MACO, mannose-modified ACO). Microscopic observation shows that MACO is a rod-shaped lipid roll with a particle size of 550 nanometers; the average particle size detected by DLS is 620 nanometers, the zeta potential is 15 mV, and the encapsulation efficiency is about 95%. In vitro experiments showed that immune cells BMDC uptake MACO in the form of recept...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com