Swine fever and porcine pseudorabies virus bivalent subunit vaccine and preparation method thereof
A technology of porcine pseudorabies virus and swine fever virus, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of increased difficulty in the prevention and treatment of porcine pseudorabies, easy secondary diseases, immune system damage, etc., to reduce artificial Cost, simple preparation method, strong effect of antigen immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
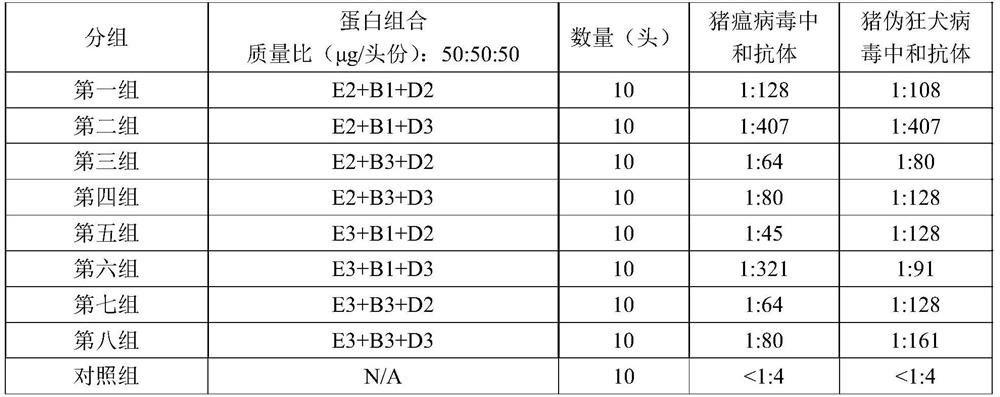
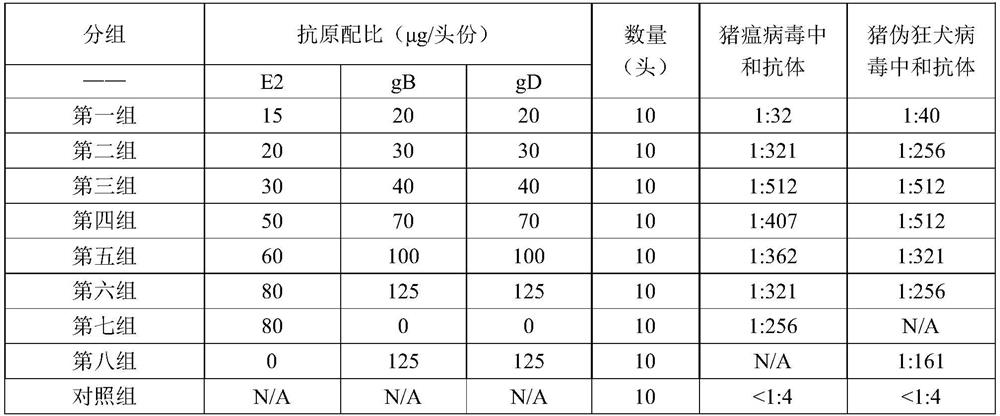
[0043]Example 2 Determination of the Antigen Ratio of Classical Swine Fever and Porcine Pseudorabies Virus Double Subunit Vaccine
[0044] In order to determine the effective ratio of classical swine fever virus antigen and porcine pseudorabies virus antigen, the ratio was adjusted according to different antigen contents, mixed with ISA 201VG and emulsified, and 8 groups of vaccine samples were prepared for mouse immunization experiments in the laboratory.
[0045] 1. Vaccine preparation: After measuring the concentration of CSFV-E2, PRV-gB and PRV-gD proteins, according to the protein content in Table 1, emulsify with sterile 201 adjuvant (purchased from SEPPIC company) to prepare a double subunit vaccine, place Store at 4°C until use.
[0046] 2. For the determination of immunogenicity, 90 healthy KM mice, weighing about 20 g, were randomly divided into 9 groups. Groups 1 to 8 were immunized with vaccines with different antigen ratios, and 10 non-immunized mice were set as ...
Embodiment 3
[0051] The immunoprotective effect of embodiment 3 classical swine fever, porcine pseudorabies virus dual subunit vaccine
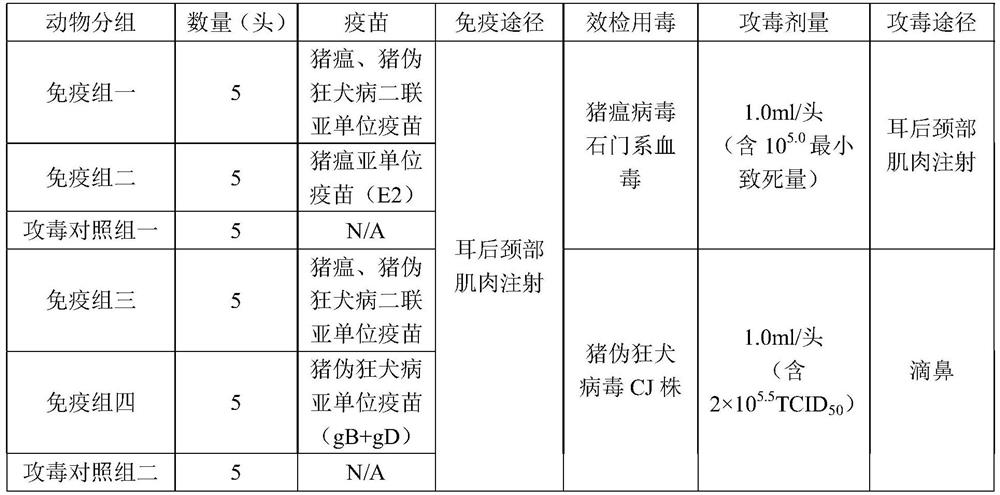
[0052] Thirty healthy susceptible pigs aged 3 to 4 weeks were used, grouped according to Table 3, and fed under the same conditions. Fourteen days after the first immunization, the second immunization was carried out in the same way and at the same dose, and serum neutralizing antibody was detected 14 days after the second immunization. 5 pigs in the immunization group and 5 pigs in the control group were intramuscularly injected with 1.0ml of CSFV Shimen blood virus (containing 10 5.0 The minimum lethal dose) was continuously observed for 16 days; 5 pigs in the immunization group and 5 pigs in the control group were inoculated with CJ strain of porcine pseudorabies virus (containing 2×10 5.5 TCID 50 ) 2.0ml, 1.0ml / nostril, observed continuously for 14 days.
[0053] Table 3 Test grouping
[0054]
[0055] Note: "N / A" (Not Available) means not appl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com